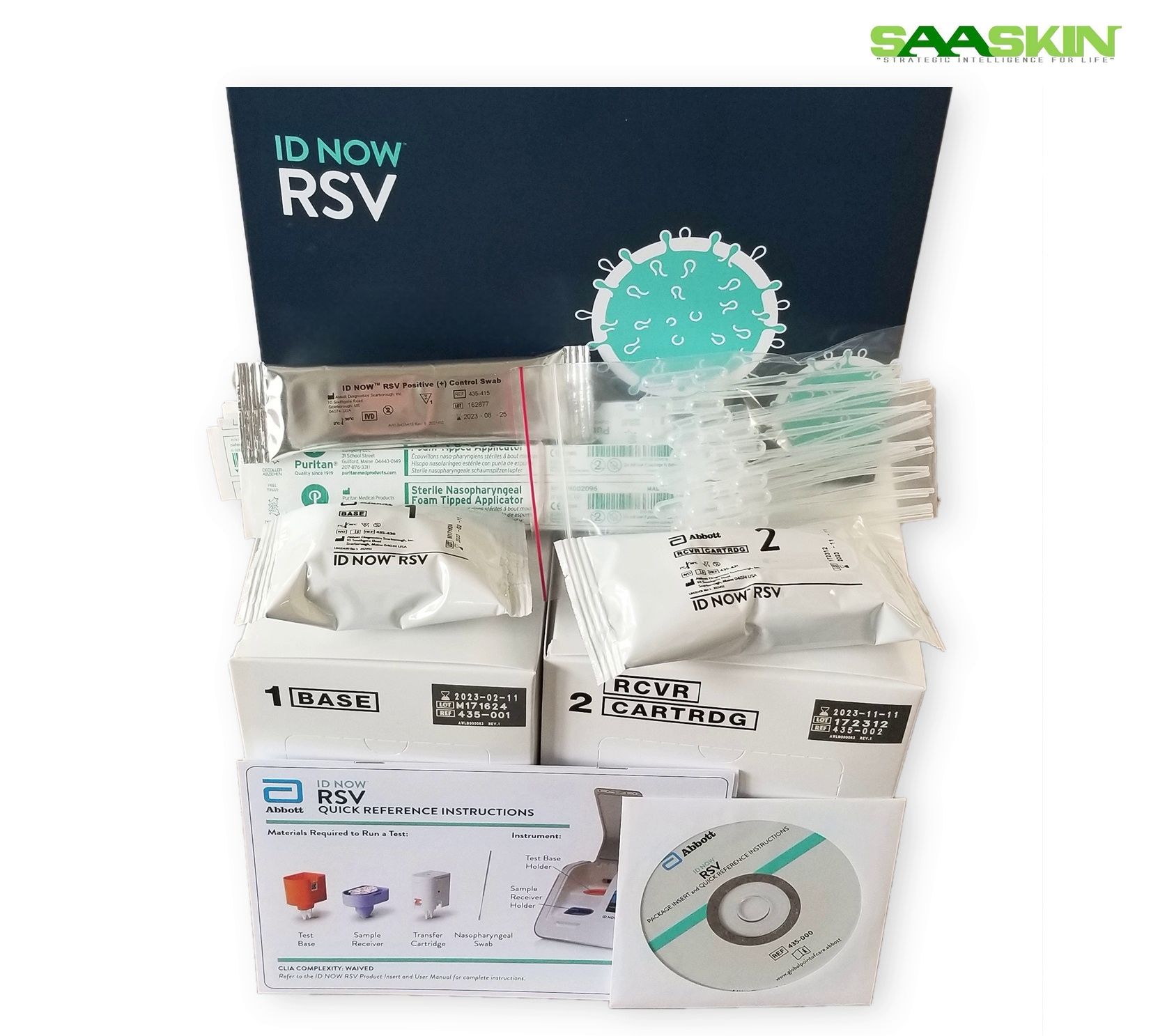
ID NOW RSV
Product Details:
- Dimension (L*W*H) 19.2 x 19.0 x 15.2 cm
- Application Clinical Diagnostics
- Feature Rapid Detection, CLIA-waived, Easy to Use
- Operation Mode Fully Automated
- Equipment Type RSV Molecular Diagnostic Instrument
- Size Compact
- Attributes Rapid, Point-of-care, User-friendly Interface
- Click to View more
X
ID NOW RSV Price And Quantity
- 15°C to 30°C
- USB, On-screen Display
- LCD Touchscreen
- 1 Sample per Run
- 13 Minutes
- Healthcare Professionals, Trained Operators
- Nasopharyngeal Swab or Aspirate
- Built-in Procedural Controls
- Available via USB
ID NOW RSV Product Specifications
- Detection of Respiratory Syncytial Virus (RSV)
- White/Blue
- Analyzer, Power Adapter, Operator Manual
- 25W
- Square
- Automatic
- AC Power Adapter
- No
- 100-240V
- Hospitals, Laboratories, Clinics
- High (95%)
- Low
- Single Sample Testing
- Plastic, Electronic Components
- Store in Cool, Dry Place
- Qualitative Detection of RSV RNA
- Yes
- New
- RSV Molecular Diagnostic Instrument
- Rapid, Point-of-care, User-friendly Interface
- Compact
- Isothermal Nucleic Acid Amplification
- Yes
- Clinical Diagnostics
- Fully Automated
- Rapid Detection, CLIA-waived, Easy to Use
- 19.2 x 19.0 x 15.2 cm
- 15°C to 30°C
- USB, On-screen Display
- LCD Touchscreen
- 1 Sample per Run
- 13 Minutes
- Healthcare Professionals, Trained Operators
- Nasopharyngeal Swab or Aspirate
- Built-in Procedural Controls
- Available via USB
ID NOW RSV Trade Information
- 1000 Unit Per Day
- Week
Product Description
| Brand | Abbott |
| Sample Type | Nasopharyngeal |
| Pack Size | 24T |
| CAT No | 435000 |
ID NOW RSV delivers molecular RSV results in 13 minutes or less on our unique ID NOW platform. Traditional laboratory methods and rapid antigen testing for RSV diagnosis have considerable shortcomings in terms of turnaround time or performance.1,2 ID NOW RSV detects 25% more true positives than Rapid Antigen Detection Tests (RADTs)3,4 and allows you to make real-time clinical decisions which impact patient care.
Rapid RSV Detection at the Point of Care
With the ID NOW RSV, healthcare professionals can quickly identify RSV infections in patients, delivering results within 13 minutes. This swift turnaround time facilitates prompt clinical decisions and timely patient management, especially crucial during peak respiratory illness seasons.
Easy Operation with User-Friendly Design
Featuring an intuitive LCD touchscreen and fully automated operation, the ID NOW RSV ensures ease of use for trained operators. The device offers clear on-screen instructions and streamlined workflows, making it accessible for hospitals, laboratories, and clinics without sacrificing accuracy or reliability.
Portable and Efficient Diagnostic Solution
Engineered for portability and efficiency, the ID NOW RSV's compact and lightweight design makes it suitable for a variety of point-of-care settings. Its low power consumption and internal quality controls provide convenience and peace of mind, while support for USB software updates ensures the device stays current.
FAQ's of ID NOW RSV:
Q: How does the ID NOW RSV detect Respiratory Syncytial Virus (RSV)?
A: The ID NOW RSV utilizes isothermal nucleic acid amplification technology to qualitatively detect RSV RNA from nasopharyngeal swab or aspirate samples. The built-in procedural controls ensure high accuracy (95%) in every test run.Q: What is the process for running a sample on the ID NOW RSV?
A: First, collect a nasopharyngeal swab or aspirate and insert it into the analyzer as instructed in the Operator Manual. The device will automatically process the sample and display the result on the LCD touchscreen within approximately 13 minutes.Q: Who can operate the ID NOW RSV instrument?
A: The ID NOW RSV is intended for use by healthcare professionals and trained operators in clinical environments such as hospitals, laboratories, and clinics. No advanced technical skills are necessary due to its fully automated, user-friendly interface.Q: What power requirements does the ID NOW RSV have?
A: The device operates with an AC power adapter, compatible with 100-240V supply, and consumes 25W of power, making it suitable for standard clinical infrastructure.Q: Where should the ID NOW RSV be used and stored?
A: This portable instrument is ideal for use at hospitals, clinics, and laboratories. For optimal function and longevity, the ID NOW RSV should be stored in a cool, dry place, and operated at temperatures between 15C and 30C.Q: What are the main benefits of using the ID NOW RSV in a clinical setting?
A: ID NOW RSV offers rapid, accurate detection of RSV at the point of care, helping clinicians make quick treatment decisions. Its compact design, ease of use, and CLIA-waived status support efficient workflows and improved patient outcomes.Q: How do I update the software on the ID NOW RSV?
A: Software updates for the ID NOW RSV can be easily carried out via USB, ensuring your analyzer remains up-to-date with the latest features and improvements.Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
Other Products in 'Medical POCT' category
 |
SAASKIN CORPORATION PRIVATE LIMITED
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |