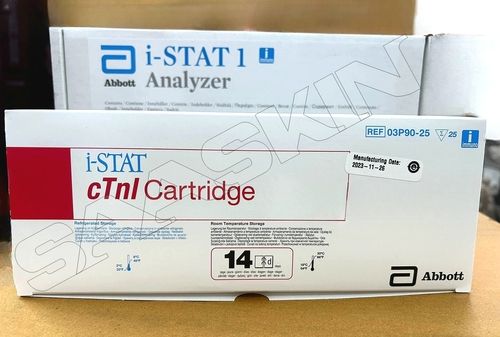
Abbott i-STAT cTnI CARTRIDGE
Product Details:
- Set Contains Individually packaged cartridges
- Color White/Off-white with coded label
- Function Detection and measurement of cardiac Troponin I in whole blood or plasma
- Size Standard i-STAT cartridge size
- Material Medical-grade plastic and microelectronics
- Accuracy High; Meets clinical sensitivity and specificity requirements
- Application Rapid cardiac marker detection in emergency and critical care
- Click to View more
X
Abbott i-STAT cTnI CARTRIDGE Price And Quantity
- Whole blood or plasma
- 28C
- Approximately 10 minutes
- Treat as biohazard after use
- Healthcare professionals
- Abbott i-STAT 1 and i-STAT Alinity analyzers
- Full traceability/unique lot number
- Up to 6 months (unopened, refrigerated)
- As specified in product IFU (typically pg/mL range)
Abbott i-STAT cTnI CARTRIDGE Product Specifications
- Silent operation
- Immunoassay (chemistry, cTnI detection)
- Not applicable (powered by i-STAT analyzer)
- Rectangular cartridge
- Store refrigerated at 28C
- Rapid cardiac marker detection in emergency and critical care
- High; Meets clinical sensitivity and specificity requirements
- New
- Yes
- Standard i-STAT cartridge size
- Medical-grade plastic and microelectronics
- Individually packaged cartridges
- White/Off-white with coded label
- Detection and measurement of cardiac Troponin I in whole blood or plasma
- Professional/clinical laboratory and POC use
- Rapid, high sensitivity, specific to cardiac Troponin I
- No
- Manual (insertion into i-STAT system)
- Single test per cartridge
- Diagnostic testing of cardiac Troponin I (cTnI)
- Cartridge: Approx. 7 cm x 2.5 cm x 1 cm
- Single-use, fast turnaround time, compatible with Abbott i-STAT analyzer
- Single-use, disposable cartridge
- Yes
- Point-of-care test cartridge
- Whole blood or plasma
- 28C
- Approximately 10 minutes
- Treat as biohazard after use
- Healthcare professionals
- Abbott i-STAT 1 and i-STAT Alinity analyzers
- Full traceability/unique lot number
- Up to 6 months (unopened, refrigerated)
- As specified in product IFU (typically pg/mL range)
Abbott i-STAT cTnI CARTRIDGE Trade Information
- 1000 Unit Per Day
- Week
Product Description
| Brand | Abbott |
| Sample Type | Whole Blood/ Plasma |
| Pack Size | 25T |
| CAT No | 03P90-25 |
With i-STAT cardiac troponin I (cTnI), healthcare professionals can obtain quantitative measurement of cardiac troponin I (cTnI) in ten minutes without leaving the patient's side.
Rapid Detection for Critical Cardiac Events
The Abbott i-STAT cTnI Cartridge facilitates quick and reliable testing of cardiac Troponin I, supporting critical decision-making in emergency and acute care settings. With results delivered in approximately 10 minutes, clinicians can diagnose cardiac events efficiently and initiate timely interventions.
Advanced Immunoassay Technology
Incorporating precise immunoassay chemistry, this cartridge ensures high sensitivity and specificity for cTnI measurement. Designed for use by healthcare professionals, it offers dependable results for rapid cardiac marker assessment using either whole blood or plasma samples.
User-Friendly and Portable Design
The cartridge is shaped for easy handling and insertion, with a standard i-STAT format and medical-grade materials. Its portability and silent operation make it suitable for point-of-care settings, aiding clinicians during high-stress situations where timely diagnosis is essential.
FAQ's of Abbott i-STAT cTnI CARTRIDGE:
Q: How is the Abbott i-STAT cTnI Cartridge used for cardiac Troponin I detection?
A: Healthcare professionals insert the single-use cartridge into an Abbott i-STAT analyzer after applying whole blood or plasma. The system processes the sample using immunoassay technology, delivering Troponin I values within about 10 minutes.Q: What are the optimal storage conditions for the i-STAT cTnI Cartridge?
A: The cartridge should be stored unopened in a refrigerated environment between 2-8C. Under these conditions, the shelf life remains up to 6 months as specified by the manufacturer.Q: When should this cartridge be used in clinical practice?
A: It is most effective in emergency rooms, critical care, and acute settings where rapid cardiac marker detection is vital for assessing suspected myocardial injury or heart attack.Q: Where can the Abbott i-STAT cTnI Cartridge be applied?
A: Designed for professional and clinical laboratory environments, this cartridge is ideal for point-of-care use in hospitals, ambulances, and other critical care facilities.Q: What are the benefits of using the i-STAT cTnI Cartridge in diagnostic testing?
A: Clinicians benefit from fast turnaround times, high diagnostic accuracy, and real-time results, enabling prompt clinical decisions during acute cardiac events.Q: How should the cartridge be disposed of after use?
A: Following diagnostic completion, the used cartridge must be treated as biohazard waste and disposed of according to established clinical safety protocols.Q: Is the cartridge suitable for both whole blood and plasma samples?
A: Yes, the Abbott i-STAT cTnI Cartridge is designed for use with either whole blood or plasma samples, providing flexibility in various diagnostic scenarios.Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
Other Products in 'Medical POCT' category
 |
SAASKIN CORPORATION PRIVATE LIMITED
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |