- Home
- About Us
-
Our Business
-
Medical IVD
- ThermoFisher Scientific Accula System Rapid Reliable RT-PCR Test
- Abbott ID NOW Instrument
- InBios Scrub Typhus Detect IgG Rapid Test
- STANDARD M nCoV SD Biosensor Real-Time Detection kit
- Thermo Fisher Taqpath Covid-19 Combo Kit
- Panbio COVID 19 Ag Rapid Test Device
- Abbott Panbio COVID Antigen Self Test
- Sure Status Covid-19 Antigen Card Test Kit
- iHealth COVID-19 Antigen Rapid Test
- Molbio Truenat COVID 19 Chip based Real Time PCR Test Kit
- STANDARD Q COVID 19 Ag
- Abbott BinaxNOW Covid-19 Rapid Antigen Card Test Kit
- MagMAX Viral Pathogen Nucleic Acid Isolation Kit
- Standard Q Covid - 19 Ag
- ID NOW COVID-19 24 Test Kit
- TRIVITRON Monkeypox Real Time PCR Test Kit
- RADI Monkeypox Virus Detection Kit
- RADI COVID-19 Detection Kit
- Hi-PCR Coronavirus (COVID-19) Multiplex Probe PCR Kit
- HIPurA Viral RNA Purification Kit
- Ultra Covid - 19 Ag
- Abbott Realtime Sars-Cov-2 Assay
-
Medical RTD
- Determine Combo 4th Generation Test Kit For Clinical
- CTK Biotech OnSite S. Typhi/Paratyphi Ag Rapid Test
- ABON hCG One Step Pregnancy Tests - For Professional Use
- Bioline CHOLERA Ag 01/0139
- SD Biosensor STANDARD Q HIV 1/2 Ab
- Roche COVID-19 At Home Test
- Premier Medical Sure Status Covid-19 Antigen Card Test Kit
- SD Biosensor STANDARD Q Influenza A/B
- Abbott Bioline ROTAVIRUS
- Abbott DETERMINE Chase Buffer
- Abbott DETERMINE HIV EARLY DETECT SET
- Abbott Bioline NOROVIRUS
- Acon Flowflex SARS-CoV-2 Antigen Rapid Test
- FIRST RESPONSE HIV 12 SYPHILIS Combo Card Test
- Abbott Bioline SALMONELLA TYPHI IgG/IgM FAST
- Abbott Bioline HAV IgG/IgM
- Abbott Bioline HIV/SYPHILIS DUO
- CTK Biotech OnSite HAV IgM Rapid Test
- CTK Biotech OnSite H. pylori Ab Combo Rapid Test
- CTK Biotech OnSite Typhoid IgG/IgM Rapid Test
- Abbott Bioline INFLUENZA ULTRA
- Bioline Rotavirus
- Bioline DENGUE IgM CAPTURE ELISA
- Erba Mannheim ErbaQik HCV Test Card
- OraSure Technologies ORAQUICK HCV RAPID ANTIBODY TEST
- Abbott Bioline HCV
- Abbott Bioline SYPHILIS 3.0
- Abbott Bioline MALARIA Ag P.f/P.v
- OraSure Technologies ORAQUICK IN-HOME HIV TEST
- Abbott DETERMINE HIV EARLY DETECT
- Abbott Panbio COVID-19 Antigen Self Test
- iScript Reverse Transcription Supermix
- Bioline TB Ag MPT64
- Thermo Fisher Scientific Thermo Scientific SickleScreen Assay Kit (120T)
- Acro Biotech JusChek Alcohol (ALC) Rapid Test Cassette
- Abbott Bioline STREP A
- Abbott Bioline INFLUENZA Ag A/B/A (H1N1)
- Roche SARS-CoV-2 Rapid Antigen Test.
- CTK Biotech OnSite Chikungunya IgM Combo Rapid Test
- Bioline Malaria Ag P.f Pan
- CTK Biotech OnSite FOB-Hi Rapid Test
- CTK Biotech OnSite RF Rapid Test
- CTK Biotech OnSite Leptospira IgG/IgM Combo Rapid TestTest
- SD Biosensor STANDARD Q HIV/Syphilis Combo
- SD Biosensor STANDARD Q Leptospira IgM/IgG
- CTK Biotech TRUSTline Scrub typhus IgG/IgM Rapid Test
- Abbott DETERMINE HIV-1/2 Set
- SD Biosensor STANDARD Q COVID/Flu Ag Combo
- SD Biosensor STANDARD Q Dengue IgM/IgG
- SD Biosensor STANDARD Q Malaria P.F/P.V Ag
- Abbott Panbio COVID-19 Ag Rapid Test Device (Nasopharyngeal)
- SD Biosensor STANDARD Q Filariasis Ag
- SD Biosensor STANDARD Q HCV Ab
- Acro Biotech JusChek COT200 Rapid Test Cassette (Urine)
- Acro Biotech JusChek COT10 Rapid Test Cassette (Whole Blood/ Serum/ Plasma)
- Abbott Bioline LEGIONELLA Ag
- SD Biosensor STANDARD Q COVID-19 IgM/IgG Combo
- Erba Mannheim ErbaQik Malaria Pf/Pan Ag (HRP2/Pan pLDH)
- Erba Mannheim ErbaQik Sickle Cell Test Card
- Abbott Bioline DENGUE NS1 Ag
- CTK Biotech TRUSTline Filariasis IgG/IgM Rapid Test
- Bioline Dengue Duo
- SD Biosensor STANDARD Q Dengue Ag+Ab Duo
- First Response SyphiliS Anti-TP CardTest
- SD Biosensor STANDARD Q Malaria P.F/Pan Ag
- InBios Dengue NS1 Detect Rapid Test
- Abbott ABON COT One Step Cotinine Test Device Urine
- Abbott Bioline MALARIA Ag P.f/P.f/P.v
- ACON hCG (Urine)
- InBios Chagas Detect Plus Rapid Test
- First Response Premier MedicalSyphilis Anti TP Card Test
- Acro Biotech JusChek Multi - Drug 10 Drugs Rapid Test Cassette (Urine)
- Abbott Bioline LEPTOSPIRA IgG/IgM
- Abbott Bioline TB Ag MPT64
- Premier Medical First Response HIV 1+2/Syphilis Combo Card Test
- Abbott Bioline TSUTSUGAMUSHI
- BinaxNOW LEGIONELLA URINARY ANTIGEN CARD
- Bioline DENGUE NS1 Ag
- Premier Medical First Response Malaria Ag pLDH/HRP2 Combo Card Test
- Erba Mannheim ErbaQik Malaria Pan Antigen test (Pan pLDH)
- Erba Mannheim ErbaQik Syphilis Test Card
- Erba Mannheim ErbaQik HBsAg Test Card
- Abbott CheckNOW HIV SELF TEST
- SD Biosensor STANDARD Q COVID-19 Ag
- Abbott DETERMINE HIV-1/2 Ag/Ab Combo
- Abbott DETERMINE HBsAg 2
- Abbott Bioline DENGUE IgG/IgM WB
- Premier Medical First Response Syphilis Anti TP Card Test
- Abbott Bioline ONCHOCERCIASIS IgG4
- Erba Mannheim ErbaQik Dengue Duo
- Erba Mannheim ErbaQik Dengue NS1 Ag
- Erba Mannheim ErbaQik Dengue IgG/IgM
- Abbott DETERMINE HIV-1/2
- Erba Mannheim ErbaQik Malaria Pf/Pv Ag (HRP2/Pv pLDH)
- Abbott DETERMINE SYPHILIS TP SET
- Abbott BinaxNOW G6PD
- Abbott PANBIO COVID-19/FLU A&B RAPID PANEL (NASOPHARYNGEAL)
- Abbott Bioline EV71 IgM
- Acro Biotech JusChek Alcohol (ALC) Rapid Test Dipstick
- Abbott Bioline HANTAAN VIRUS
- Abbott NMP22 BLADDERCHEK
- FIRST RESPONSE HIV 1 - 2.0 CARD TEST
- Abbott Bioline CHLAMYDIA
- Abbott BINAXNOW MALARIA
- Premier Medical FIRST RESPONSE HIV 1-2.O CARD TEST (SELF TEST)
- CTK Biotech OnSite TORCH Panel Rapid Test
- Acro Biotech JusChek Multi-Drug 6 Drugs Rapid Test Cassette (Urine)
- CTK Biotech OnSite Syphilis Ab Rapid Test (strip) CE
- Abbott DETERMINE SYPHILIS TP
- Abbott Bioline ONCHO/LF IgG4 BIPLEX
- SD Biosensor STANDARD Q Chikungunya IgM/IgG
- Abbott Bioline H. PYLORI Ab
- SD Biosensor STANDARD i-Q COVID/Flu Ag Combo Test
- Abbott Bioline LEISHMANIA Ab
- InBios CL Detect Rapid Test for Cutaneous Leishmaniasis
- Abbott DETERMINE TB LAM Ag
- Abbott Bioline MALARIA Ag P.f/Pan
- Roche Roche cardiac Troponin T sensitive test (visual)
- Abbott Bioline HBsAg
- Abbott Bioline Filariasis Test Strip
- Abbott BINAXNOW COVID-19 Ag CARD
- SD Biosensor STANDARD Q Dengue NS1 Ag
- Abbott BINAXNOW STREP A
- SD Biosensor Ultra Covid-19 Ag
- CTK Biotech Onsite HEV IgM Rapid Test CE
- Abbott Bioline HAT 2.0
- CTK Biotech OnSite Dengue IgG/IgM 3.0 Combo Rapid Test CE
- Abbott Bioline RSV
- Abbott SURESTEP URINE DRUG TEST E-Z SPLIT KEY CUP
- Abbott Determine HBsAg 2 SET
- CTK Biotech OnSite Dengue Ag Rapid Test CE
- Thermo Fisher Scientific Thermo Scientific SickleScreen Assay Kit (1T)
- CTK Biotech Onsite Typhoid IgG/IgM Combo Rapid Test CE
- Acro Biotech JusChek COT20 Rapid Test (Oral Fluid)
- SD Biosensor STANDARD Q Ultra Dot HCV
- CTK Biotech OnSite H. pylori Ag Rapid Test
- Abbott BINAXNOW LEGIONELLA URINARY ANTIGEN CARD
- HemoTypeSC HemoTypeSC Sickle Cell Disease
- Acon ACON hCG (Urine)
- SD Biosensor STANDARD Q Ultra-Dot HIV
- Acro Biotech JusChek Alcohol (ALC) Rapid Test Dipstick (Saliva)
- ABON Syphilis Ultra Rapid Tests (Whole Blood Serum Plasma)
- Abbott Bioline DENGUE DUO
- CTK Biotech OnSite Toxo IgG/IgM Combo Rapid Test
- First Response Malaria Ag. P.f. P.v. Card Test
- FIRST RESPONSE Malaria Antigen P. faciparum(HRP2) Card Test
- CTK Biotech OnSite Troponin I Combo Rapid Test
- CTK Biotech OnSite HAV IgG/IgM Rapid Test CE
- Bioline Malaria Ag P.f Pv
- Abbott PANBIO HIV SELF TEST
- Abbott Bioline ROTA/ADENO
- FIRST RESPONSE Malaria Ag. pLDH HRP2 Combo Card Test
- Roche Cardiac Trop T Sensitive Test
- Bioline HCV
- Bioline HIV 1 2 3.0
- Determine HIV-1 2
- Premier Medical FIRST RESPONSE Malaria Antigen P. faciparum(HRP2) Card Test
- Binaxnow Streptococcus Pneumoniae
- Abbott Bioline RUBELLA IgG/IgM
- Abbott Bioline H. PYLORI Ag
- SD Biosensor STANDARD Q RSV Ag
- Abbott Bioline LYMPHATIC FILARIASIS IgG4
- SD Biosensor STANDARD Q HBsAg
- Abbott BINAXNOW STREPTOCOCCUS PNEUMONIAE ANTIGEN CARD
- SD Biosensor STANDARD Q TSUTSUGAMUSHI IgM/IgG
- Abbott Bioline HIV 1/2 3.0
- SD Biosensor STANDARD Q Malaria P.f Ag
- Determine HBsAg
- Abbott Bioline INFLUENZA ANTIGEN
- Premier Medical First Response Malaria Ag pf/pv Card Test
- OnSite Duo Dengue Ag IgG IgM Rapid Test
- STANDARD Q Tsutsugmushi IgM IgG
- STANDARD Q Malaria P.f Pan Ag
- STANDARD Q Dengue Ag Ab Duo
- STANDARD Q Malaria PF PV Ag
- Standard Q Filariasis Ag
- STANDARD Q Dengue IgM IgG
- OnSite FOB-Hi Rapid Test
- OnSite HEV IgM Rapid Test
- OnSite H.Pylori Ag Rapid Test
- OnSite HAV IgM Rapid Test
- OnSite HAV IgG IgM Rapid Test
- OnSite Leptospira IgG IgM Combo Rapid Test
- TRUST line Filariasis IgG IgM Combo Rapid Test
- OnSite S.Typhi Paratyphi Ag Rapid Test
- OnSite Typhoid IgG IgM Rapid Test
- OnSite Dengue IgG IgM Combo Rapid Test
- OnSite Dengue Ag Rapid Test
- STANDARD Q HIV 1-2 Ab Test
- STANDARD Q Chikungunya IgM IgG
- STANDARD Q Ultra-Dot HCV
- STANDARD Q Hbsag
- STANDARD Q Ultra-Dot HIV
- STANDARD Q HCV Ab
- STANDARD Q Dengue NS1 Ag
- OnSite H. Pylori Ab combo Rapid Test
- STANDARD Q - HIV Syphilis Combo
- OnSite Toxo IgG IgM Combo Rapid Test
- Standard Q Lepospira Igg Igm
- N SURE One step Pragnancy Detention Test
- OnSite RF Rapid Test
- OnSite TORCH Panel Rapid Test
- Dengue Day 1 Test
- Advantage MAL Card
- CTK TRUSTline Scrub typhus IgG IgM Rapid Test
- HCV TRI DOT
- HIV TRI DOT
- Hepa card
- Abbott Bioline LEPTOSPIRA IgM
- Bioline CHAGAS Ab
- PANBIO COVID-19 IgG/IgM RAPID TEST DEVICE
- STANDARD Q COVID-19 Ag
- CTK Biotech OnSite Duo Dengue Ag-IgG/IgM Rapid Test CE
- Abbott PANBIO HIV VERIFICATION TEST
- ORAQUICK IN-HOME HIV TEST (Community Version)
- ORAQUICK IN-HOME HIV TEST (Pharmacy Version)
- ORAQUICK IN-HOME HIV TEST (US FDA Version)
- Abbott BINAXNOW INFLUENZA A&B CARD
- Abbott Bioline MALARIA Ag P.f (HRP2/pLDH)
- Abbott NxTek ELIMINATE MALARIA Pf
- Abbott BINAXNOW RSV CARD
- Abbott TESTPACK PLUS WITH OBC STREP A
- Abbott Bioline ZIKA IgM
- Erba Mannheim ErbaQik HIV 1+2 Test Card
-
Medical ELISA
- Calbiotech CEA ELISA
- Calbiotech Beta2 Microglobulin ELISA
- Calbiotech Brucella IgG ELISA
- Calbiotech AFP ELISA
- Scrub Typhus Detect IgM ELISA Kit
- Calbiotech Free Beta hCG ELISA
- Calbiotech hCG ELISA
- Calbiotech Free Triiodothyronine (FT3) ELISA
- Calbiotech DHEA-S ELISA
- CTK Biotech TRUSTwell Vitamin-D (Total) ELISA
- Calbiotech dsDNA IgG ELISA
- SD Biosensor STANDARD E TB-Feron Tubes 300
- Calbiotech CA 15-3 ELISA
- Calbiotech CMV IgG ELISA
- CTK Biotech RecombiLISA HAV IgM ELISA CE
- SD Biosensor STANDARD E Dengue IgM Capture ELISA
- CTK Biotech RecombiLISA Leptospira IgM ELISA
- Calbiotech Neonatal 17(OH) Progesterone ELISA
- Calbiotech Troponin I ELISA
- Calbiotech Luteinizing Hormone (LH) ELISA
- Calbiotech Immunoglobulin E (IgE) ELISA
- Calbiotech SSA IgG ELISA
- Calbiotech PSA ELISA
- Calbiotech Bio Intact PTH ELISA
- Calbiotech VZV IgG ELISA
- Calbiotech Thyroxine (T4) ELISA
- InBios Scrub Typhus Detect IgM ELISA Kit
- Calbiotech Neonatal TSH ELISA
- Abbott Bioline DENGUE NS1 Ag ELISA
- InBios DENV Detect NS1 ELISA
- Calbiotech Brucella IgM ELISA
- Calbiotech Dengue Virus IgM ELISA
- Calbiotech Sm IgG ELISA
- Calbiotech ANA Screen IgG ELISA
- Calbiotech Rubella IgG ELISA
- Calbiotech Mitochondrial (MA) Ab ELISA
- Calbiotech Insulin ELISA
- Calbiotech Scl-70 ELISA
- Standard E Dengue Elisa Analyzes IgM Test Kit
- SD Standard E Dengue IgG Elisa Analyzes Test Kit
- Standard E Dengue NS1 Elisa Analyzes Test Kit
- Calbiotech Cardiolipin IgM ELISA
- Calbiotech CMV IgM ELISA
- Calbiotech Free PSA ELISA
- CTK Biotech TRUSTwell Free T4 (fT4) ELISA kit
- Calbiotech Folate ELISA
- Calbiotech HE4 ELISA
- Calbiotech Ferritin ELISA
- Erba Mannheim System Pack LDL-Cholesterol with Calibrator
- Calbiotech CA-125 ELISA
- Calbiotech VZV IgM ELISA
- Calbiotech Cardiolipin IgG, IgM, IgA ELISA
- Calbiotech RNP/Sm Autoantibody ELISA
- Calbiotech 25(OH) Vitamin D ELISA
- Calbiotech Vitamin B12 ELISA
- Calbiotech C-Peptide ELISA
- Calbiotech Cardiolipin IgA ELISA
- Calbiotech Thyroid Peroxidase (TPO) IgG ELISA
- Calbiotech Cardiolipin IgG ELISA
- CTK Biotech TRUSTwell Ferritin ELISA
- Calbiotech SSB IgG ELISA
- Calbiotech Thyroglobulin Ab (TG) ELISA
- Calbiotech Intact PTH (Parathyroid Hormone) ELISA
- Calbiotech CA 19-9 ELISA
- Panbio Dengue IgM Capture ELISA
- Panbio Leptospira IgM Elisa
- Panbio DENGUE Ig CAPTURE ELISA
- Panbio DENGUE EARLY ELISA
- RecombiLISA Dengue Ag ELISA Kit
- TRUSTwell CHIK IgM ELISA Kit
- TRUSTwell HBsAg ELISA Kit
- TRUSTwell HIV Ag-Ab ELISA Kit
- TRUSTwell HIV 1 2 Ab ELISA Kit
- TRUSTwell Dengue IgG ELISA Kit
- Scrub Typhus Detect IgG ELISA System
- CHIKjj Detect IgG ELISA Kit
- Filaria Detect IgG4 ELISA
- ZIKV Detect 2.0 IgM Capture ELISA
- Bioline DENGUE NS1 Ag ELISA
- RecombiLISA HAV IgM ELISA Kit
- RecombiLISA Total T3 Elisa Kit
- RecombiLISA Total T4 ELISA Kit
- RecombiLISA TSH ELISA kit
- RecombiLISA Leptospira IgM ELISA Kit
- TRUSTwell Dengue IgM ELISA Kit
- TRUSTwell Syphilis Ab kit
- TRUSTwell HCV IgG ELISA Kit
- STANDARD E TB-Feron Tubes 200
- STANDARD E TB-Feron Tubes 300
- J Mitra ELISA Reader
- J Mitra ELISA Washer
- DENV Detect NS1 ELISA
- InBios DENV Detect IgM Capture ELISA
- CHIKjj Detect IgM ELISA Kit
- RecombiLISA HEV IgM ELISA Kit
- CTK Biotech TRUSTwell CHIK IgM ELISA CE
- Chlamydia pneumonia IgG ELISA
- Chlamydia pneumonia IgM ELISA
- Chlamydia trachomatis IgA ELISA
- Chlamydia trachomatis IgG ELISA
- Chlamydia trachomatis IgM ELISA
- CTK Biotech TRUSTwell COVID-19 IgG ELISA
- Abbott Bioline DENGUE IgG CAPTURE ELISA
- Abbott BIOLINE DENGUE IgM CAPTURE ELISA
- Abbott Panbio DENGUE EARLY ELISA
- Abbott Panbio DENGUE IgG CAPTURE ELISA
- Abbott Panbio DENGUE IgG INDIRECT ELISA
- Abbott Panbio DENGUE IgM CAPTURE ELISA
- Calbiotech Dengue Virus IgG ELISA
- CTK Biotech TRUSTwell Dengue Ag ELISA
- CTK Biotech TRUSTwell Dengue IgG ELISA CE
- CTK Biotech TRUSTwell Dengue IgM ELISA CE
- SD Biosensor STANDARD E Dengue IgG Capture ELISA
- SD Biosensor STANDARD E Dengue NS1 Ag ELISA
- Calbiotech EBV-VCA IgG ELISA
- Calbiotech EBV-VCA IgM ELISA
- Calbiotech AMH ELISA
- Calbiotech Follicle Stimulating Hormone (FSH) ELISA
- Calbiotech Prolactin ELISA
- CTK Biotech TRUSTwell Anti-Mullerian hormone (AMH) ELISA
- Calbiotech 17(OH) Progesterone ELISA
- Calbiotech Androstenedione ELISA
- Calbiotech Estradiol ELISA
- Calbiotech Progesterone ELISA
- Calbiotech Growth Hormone ELISA
- Calbiotech H. Pylori IgA ELISA
- Calbiotech H. Pylori IgG ELISA
- Calbiotech H. Pylori IgM ELISA
- CTK Biotech RecombiLISA HEV IgM ELISA CE
- CTK Biotech TRUSTwell HBsAg ELISA
- CTK Biotech TRUSTwell HCV Total Ab ELISA
- Calbiotech HSV-1 IgG ELISA
- Calbiotech HSV-1 IgM ELISA
- Calbiotech HSV-2 IgG ELISA
- Calbiotech HSV-2 IgM ELISA
- Athenese-Dx TRUSTwell HIV 1+2 Ab ELISA
- CTK Biotech TRUSTwell HIV Ag-Ab ELISA
- Calbiotech C-Reactive Protein ELISA
- Abbott Panbio LEPTOSPIRA IgM ELISA
- CTK Biotech TRUSTwell Malaria Ag ELISA
- Calbiotech Measles IgG ELISA
- Calbiotech Measles IgM ELISA
- Calbiotech Mumps IgG ELISA
- Calbiotech Mumps IgM ELISA
- Calbiotech Mycoplasma IgG ELISA
- Calbiotech Mycoplasma IgM ELISA
- Calbiotech Neonatal Galactose ELISA
- Calbiotech Neonatal Maple Syrup Urine Disease (N-MSUD) ELISA
- Calbiotech Neonatal PKU ELISA
- Abbott Bioline ONCHOCERCIASIS IgG4 ELISA
- Calbiotech Rubella IgM ELISA
- Calbiotech Salmonella IgG ELISA
- Calbiotech Salmonella IgM ELISA
- Calbiotech Cortisol ELISA
- Calbiotech Treponima pallidum IgG ELISA
- CTK Biotech TRUSTwell Syphilis Ab ELISA CE
- SD Biosensor STANDARD E TB-Feron Tubes 200
- Calbiotech Free Thryoxine (FT4) ELISA
- Calbiotech Triiodothyronine (T3) ELISA
- Calbiotech TSH ELISA
- CTK Biotech TRUSTwell Free T3 ELISA Kit
- CTK Biotech TRUSTwell Total T3 Elisa Kit
- CTK Biotech TRUSTwell Total T4 ELISA Kit
- CTK Biotech TRUSTwell TSH ELISA Kit
- Calbiotech Cotinine ELISA
- Calbiotech Toxoplasma IgG ELISA
- Calbiotech Toxoplasma IgM ELISA
- Qiagen QuantiFERON-TB Gold Plus Blood Collection Tubes
-
Medical FIA
- Standard F B-hCG FIA
- ichroma testoste1 Test
- STANDARD F Strep A Ag FIA
- Boditech ichroma Vitamin D Neo
- Wondfo Finecare PSA Rapid Quantitative Test
- Getein CK-MB/cTnI Fast Test Kit
- Standard F Dengue NS1 Ag FIA
- Finecare Fia Meter lll Plus
- Getein Novel Coronavirus (2019-nCoV) IgM/IgG Antibody Fast Test Kit
- ichroma D Dimer Test
- Wondfo Finecare beta hCG Rapid Quantitative Test
- Getein Prog Fast Test Kit
- wondfo Finecare CEA Rapid Quantitative Test
- STANDARD F Dengue NS1 Ag FIA
- Acon FIAflex cTnI FIA Test
- Wondfo Finecare Testosterone Rapid Quantitative Test
- Wondfo Finecare FIA Meter III Plus
- ichroma Creatine Kinase Muscle Brain (CK-MB)
- STANDARD F T4
- Wondfo Finecare Dengue NS1 Ag Test
- Boditech AFIAS Thyroid Stimulating Hormone (TSH) Plus
- Wondfo Finecare IL-6 Rapid Quantitative Test
- Boditech ichroma II Controls
- Standard F FT4
- Wondfo Finecare T4 Rapid Quantitative Test
- Standard F HS-CRP FIA
- Finecare FIA Meter ll Plus SE
- Strandard F U-Albumin FIA
- Wondfo Finecare cTn I Rapid Quantitative Test
- Wondfo Finecare One Step MAU Rapid Quantitative Test
- Wondfo Finecare AFP Rapid Quantitative Test
- Wondfo Finecare FIA Meter II Plus SE
- Getein fPSA Fast Test Kit
- Arkray AutoChem MultiCheck FIA Analyzer
- SD Biosensor STANDARD F2400 Analyzer
- Boditech AFIAS Troponin I (Tn-I) Plus
- Getein 1100 Immunofluorescence Quantitative Analyzer
- SD Biosensor STANDARD F200 Analyzer
- wondfo Finecare CK-MB Rapid Quantitative Test
- Wondfo Finecare FSH Rapid Quantitative Test
- Wondfo Finecare TSH Rapid Quantitative Test
- Getein HCG+beta Fast Test Kit
- ichroma Anti-CCP Plus
- Getein IL-6 Fast Test Kit
- ichroma Troponin I (Tn-I)
- Wondfo Finecare NT-proBNP Rapid Quantitative Test.
- STANDARD F T3 FIA
- Wondfo Finecare FIA Meter Plus
- Wondfo Finecare FPSA Rapid Quantitative Test
- Wondfo Finecare D-Dimer Rapid Quantitative Test
- Wondfo Finecare Cortisol Rapid Quantitative Test
- STANDARD F hs-CRP FIA
- ichroma Anti-CCP Test
- Wondfo Finecare CRP (C-reactive protein) Rapid Quantitative Test
- Wondfo Finecare LH Rapid Quantitative Test
- STANDARD F H. Pylori Ag FIA
- ichroma Total IgE Test
- Ichroma Tn-I Plus Test
- STANDARD F fT4
- Ichroma Vitamin D Test
- STANDARD F TSH-II FIA
- ichroma NT-proBNP Test
- Boditech I Chroma III Diagnostic Immuno Analyzer
- ichroma Test
- ichroma PRL Test
- STANDARD F TSH FIA
- Ichroma II Immuno Analyzer
- Standard F100 Analyzer
- SD Biosensor Standard F200 Analyzer
- Standard F2400 Analyzer
- ichroma Progesterone Test
- ichroma PSA Test
- ichroma HbA1c Test
- Ichroma T3 Test
- Ichroma T4 Test
- ichroma Beta-HCG Test
- ichroma TSH Test
- Standard F PCT FIA
- Standard F Vitamin D FIA
- Standard F Dengue IgM IgG FIA
- Standard F Covid-19 Ag FIA
- Standard F iFOB FIA
- Ichroma AMH Test
- ichroma FSH Test
- ichroma LH Test
- Standard F Chikungunya IgM IgG FIA
- Ichroma CRP Test
- Standrard F HbA1c
- Standard F RSV Ag FIA
- Standard F S.Pneumoniae Ag FIA
- Standard F Strep A Ag FIA
- Standard F Legionella Ag FIA
- Standard F D-Dimer FIA
- Standard F NT-ProBNP FIA
- Standard F LH FIA
- Standard F TSH FIA
- Standard F T4
- Standard F CRP
- Standard F CK-MB FIA
- Standard F TnI FIA
- ichroma PCT Test
- Getein 1600 Immunofluorescence Quantitative Analyzer
- Boditech AFIAS-1
- Boditech AFIAS-6
- Boditech ichroma i-Chamber
- Boditech ichroma II Fluorescence Immunoassay Analyzer
- Boditech ichroma III Fluorescence Immunoassay Analyzer
- SD Biosensor STANDARD F100 Analyzer
- Wondfo Finecare 2-MG Rapid Quantitative Test
- Wondfo Finecare AMH Rapid Quantitative Test
- Wondfo Finecare Cys C Rapid Quantitative Test
- Wondfo Finecare Estradiol (E2) Rapid Quantitative Test
- Wondfo Finecare Ferritin Rapid Quantitative Test
- Wondfo Finecare fT3 Rapid Quantitative Test
- Wondfo Finecare fT4 Rapid Quantitative Test
- Wondfo Finecare H-FABP Rapid Quantitative Test
- Wondfo Finecare HbA1c Rapid Quantitative Test
- Wondfo Finecare Myo Rapid Quantitative Test
- Wondfo Finecare NGAL Rapid Quantitative Test
- Wondfo Finecare One Step cTn I/CK-MB/Myo Rapid Quantitative Test
- Wondfo Finecare PCT Rapid Quantitative Test
- Wondfo Finecare Progesterone Rapid Quantitative Test
- Wondfo Finecare Prolactin Rapid Quantitative Test
- Wondfo Finecare T3 Rapid Quantitative Test
- Wondfo Finecare Total IgE Rapid Quantitative Test
- Wondfo Finecare Vitamin D Rapid Quantitative Test
- Getein beta2-MG Fast Test Kit
- Getein 25-OH-VD Fast Test Kit
- Getein AFP Fast Test Kit
- Getein AMH Fast Test Kit
- Getein Anti-CCP Fast Test Kit
- Getein Anti-HBs Fast Test Kit
- Getein Anti-HCV Fast Test Kit
- Getein Anti-HIV Fast Test Kit
- Getein Anti-TP Fast Test Kit
- Getein ASO Fast Test Kit
- Getein BNP Fast Test Kit
- Getein Cardiac Troponin I Fast Test Kit
- Getein CEA Fast Test Kit
- Getein CK-MB Fast Test Kit
- Getein CK-MB/cTnI/H-FABP Fast Test Kit
- Getein CK-MB/cTnI/Myo Fast Test Kit
- Getein CysC Fast Test Kit
- Getein D-Dimer Fast Test Kit
- Getein Dengue NS1 Ag Fast Test Kit
- Getein E2 Fast Test Kit
- Getein Ferritin Fast Test Kit
- Getein FSH Fast Test Kit
- Getein fT3 Fast Test Kit
- Getein fT4 Fast Test Kit
- Getein H-FABP Fast Test Kit
- Getein H. pylori Fast Test Kit
- Getein HbA1c Fast Test Kit
- Getein HBsAg Fast Test Kit
- Getein hs-CRP+CRP Fast Test Kit
- Getein hs-cTnI Fast Test Kit
- Getein LH FastTest Kit
- Getein mAlb Fast Test Kit
- Getein NGAL Fast Test Kit
- Getein NT-proBNP Fast Test Kit
- Getein PCT Fast Test Kit
- Getein PRL Fast Test Kit
- Getein RF Fast Test Kit
- Getein SAA Fast Test Kit
- Getein SAA/CRP Fast Test Kit
- Getein SARS-CoV-2 Antigen Fast Test Kit
- Getein SARS-CoV-2 Neutralizing Antibody Fast Test Kit
- Getein T3 Fast Test Kit
- Getein T4 Fast Test Kit
- Getein TnT Fast Test Kit
- Getein Total IgE Fast Test Kit
- Getein tPSA Fast Test Kit
- Getein TSH Fast Test Kit
- ichroma Alpha-Fetoprotein (AFP)
- ichroma Anti-Mllerian Hormone (AMH)
- ichroma Anti-streptolysin O (ASO)
- ichroma BNP
- ichroma Calprotectin
- ichroma Carcinoembryonic antigen (CEA)
- ichroma Cardiac Triple
- ichroma Cortisol
- ichroma COVID-19 Ab
- ichroma COVID-19 Ag
- ichroma CRP
- ichroma Cystatin C
- ichroma D-Dimer
- ichroma Dengue IgG/IgM
- ichroma Dengue NS1 Ag
- ichroma Fecal Occult Blood (iFOB) Neo
- ichroma Ferritin
- ichroma Follicle-stimulating Hormone (FSH)
- ichroma Group A Streptococcal Infection (Strep A)
- ichroma H.pylori SA
- ichroma HbA1c
- ichroma HbA1c Neo
- ichroma hsCRP
- ichroma Human Chorionic Gonadotropin (beta-hCG)
- ichroma Human Chorionic Gonadotropin (beta-hCG) Plus
- ichroma IGRA-TB Tube
- ichroma Influenza A+B
- ichroma Interleukin-6 (IL-6)
- ichroma Luteinizing Hormone (LH)
- ichroma Microalbumin
- ichroma Myoglobin
- ichroma Norovirus (Noro)
- ichroma NT-proBNP test
- ichroma PCT
- ichroma Progesterone test
- ichroma Prolactin (PRL)
- ichroma Prostate Specific Antigen (PSA)
- ichroma Rheumatoid Arthritis (RF IgM)
- ichroma Rotavirus (Rota)
- ichroma Rotavirus/Adenovirus (Rota/Adeno)
- ichroma ST2
- ichroma T3
- ichroma T4
- ichroma Thyroid Stimulating Hormone (TSH)
- ichroma Total IgE
- ichroma Toxo IgG/IgM
- ichroma Troponin I (Tn-I) Plus
- ichroma Troponin T (Tn-T)
- ichroma Vitamin D
- STANDARD F beta-hCG FIA
- STANDARD F Chikungunya IgM/IgG FIA
- STANDARD F CK-MB FIA
- STANDARD F COVID-19 Ag FIA
- STANDARD F CRP
- STANDARD F D-dimer FIA
- STANDARD F Dengue IgM/IgG FIA
- STANDARD F HbA1c
- STANDARD F iFOB FIA
- STANDARD F Legionella Ag FIA
- STANDARD F LH FIA
- STANDARD F NT-proBNP FIA
- STANDARD F PCT FIA
- STANDARD F RSV Ag FIA
- STANDARD F S. pneumoniae Ag FIA
- STANDARD F TnI FIA
- STANDARD F Tsutsugamushi IgM/IgG FIA
- STANDARD F U-Albumin FIA
- STANDARD F Vitamin D FIA
- Getein Testosterone Fast Test Kit
- Boditech ichroma Testosterone Test
-
Medical POCT
- Medtronic HMS Plus Cartridges - Blue - 2.5-4.0 mg/kg
- HemoCue Glucose 201 RT System
- SD Biosensor STANDARD GlucoNavii NFC Blood Glucose Monitoring System
- Abbott AFINION CRP Rapid Vitro Diagnostic Test
- Acon Mission Hb Hemoglobin Testing System
- Abbott CHOLESTECH LDX LIPID PROFILEGLU CASSETTE
- HemoCue Albumin 201 Control Set
- Acon On Call A1c HbAl c Test Kit
- Acon Mission HemoPro Hemoglobin Meter
- On Call Plus Glucometer Test Strips
- Erba Mannheim DekaPHAN LAURA
- Medtronic HMS Plus Cartridges - Red - 0.0-0.9 mg/kg
- URIT - 12 Hemoglobin Meter
- Roche Accu-Chek Guide Glucometer Kit
- Abbott Pima CD4 Analyser
- HemoCue Glucose 201+ System
- Medtronic ACT PLUS Disposable Test Cartridges (LR ACT)
- SD Biosensor STANDARD G6PD Test Strip
- HemoCue HbA1c 501 System.
- URIT 14G Urine Reagent Strips
- Abbott i-STAT G3+ Cartridge
- Medtronic HMS Plus Cartridges - Silver - 2.0-3.5 mg/kg
- HemoCue Hb 201+ Microcuvettes
- Abbott AFINION CRP
- Abbott i-STAT cTnI CARTRIDGE
- Abbott ID NOW COVID-19 Test Kit
- Abbott i-STAT1 Analyzer
- Abbott UroColor 10
- Roche Combur 9 Test Strip
- HemoCue Glucose 201 Microcuvettes
- HemoCue Plasma/Low Hb Microcuvettes
- Roche Combur 7 Test Strip
- HemoCue Cleaner Plus
- HemoCue HbA1c 501 Daily Check Cartridge
- AFINION ACR Rapid Test
- Acon Mission Urinalysis Strips - 2P (Glucose & Protein)
- Roche Accutrend Cholesterol Test Strips
- HemoCue WBC DIFF Microcuvettes
- Roche CoaguChek XS Plus system
- EKF Diagnostics DiaSpect T D24 Hemoglobin Microcuvettes
- COPACK Haemoglobin Colour Scale
- Insta Q96
- Medtronic HMS Plus Cartridges - White - 2.5-5.0 mg/kg
- Roche Combur 10 UX Test strip
- CLINITEK Microalbumin 2 Reagent Strips
- HemoCue Hb 301 Microcuvettes
- STANDARD GlucoNavii NFC Blood Glucose Test Strip
- EKF Diagnostics Hemo Control Hemoglobin Analyzer
- Abbott AFINION ACR
- Acon On Call MultiPro
- Roche Coagu Chek Xs Plus System
- Siemens epoc BGEM Test Card (25T)
- Abbott i-STAT EG7+ CARTRIDGE
- Medtronic HMS Plus Hemostasis Management System.
- Abbott UROMETER 120 URINE CHEMISTRY ANALYZER
- SD Biosensor STANDARD G6PD Analyzer
- Abbott i-STAT Crea Cartridge
- Roche Cobas b 123 POC system
- Roche Chemstrip 10 MD Test Strips
- Roche MICRAL-Test Strips
- ARKRAY Insulin Cooling Pouch - Duo Z for Travel
- Medtronic HMS Plus Cartridges - Tan - 1.5-3.0 mg/kg
- URIT 12 Hemoglobin Strips
- I Stat Test Cartridges
- PocketChem A1C HbA1c - Device
- Afinion 2 Rapid Multi Assay Analyzer
- Pima CD4 Analyzer
- PocketChem UA PU-4010
- Skyla HB1 POC Clinical Chemistry Auto Analyzer
- Hb 201 System
- Spotchem El Se-1520
- HemoCue HbA1c 501 System
- Abbott Afinion Hba 1 C Human Whole Blood Test Kit
- HemoCue Albumin 201 System
- HemoCue Plasma Low Hb System
- HemoCue WBC System
- Siemens EPOC Blood Analysis System with EPOC NXS Host
- HemoCue Hb 301 System
- SD Urometer 120 720 Analyzer For Laboratory
- AFIAS 6
- i-STAT 300 Handheld Blood Analyzer
- Pocketchem HemoG
- Mission Hemoglobin Hb And Test Strip
- Siemens CLINITEK Status Analyzer
- Nycocard Reader II
- Siemens Clinitek Microalbumin 2
- Accu-Chek Active blood glucose meter
- CHOLESTECH LDX ANALYZER
- Siemens Multistix 10 SG Reagent Urine Analysis Strip
- Accu-Check Test Strips
- Epoc Blood Test Strips 25T
- Afinion Lipid Panel Test1
- Sysmex CA 104
- Hemocue HbA1c 501 Test Cartridge
- HemoCue WBC DIFF 25 Microcuvettes
- Arkray TRUSTCHECK BCA
- HemoCue Cleaner
- HemoCue Hardtop Carrying Case
- HemoCue Martel Printer Kit Universal
- Roche Accu-Chek Softclix Lancing Device Kit
- HemaPrep CellaVision HemaPrep
- HemoCue Albumin 201 System.
- Arkray PocketChem BA PA-4140 Blood Ammonia Meter
- FUJIFILM DRI-CHEM NX10N
- Siemens epoc Blood Analysis System
- Wondfo Blood Gas Analyzer
- Arkray Trustcheck BPM 2.0 Digital Blood Pressure Monitor
- Abbott CHOLESTECH LDX ANALYZER
- Acon Mission Cholesterol Meter
- Acon Mission Ultra Cholesterol Monitoring System
- Roche CoaguChek XS system
- Arkray Spotchem EL SE-1520 Electrolyte Test Analyzer
- Abbott FreeStyle Optium Neo Meter
- ACON On Call Plus Glucometer
- Arkray Blood Glucose Test Meter GLUCOCARD Sigma GT-1070
- Arkray Blood Glucose Test Meter GLUCOCARD Vital GT-1050
- Roche Accu-Chek Active blood glucose meter
- Roche Accu-Chek Instant Glucometer Kit
- Roche Accu-Chek Instant S Glucometer Kit
- SD Biosensor SD CodeFree Blood Glucose Monitoring System
- Acon On Call A1c HbA1c Analysis System
- Arkray Compact Glycohemoglobin Analyzer PocketChem A1c
- EKF Diagnostics Quo-Lab HbA1c Analyzer
- Arkray Pocketchem HemoG Hemoglobin Meter
- Boditech Hemochroma PLUS Total Hemoglobin Meter
- COPACK GMBH Heamoglobin Color Scale
- EKF Diagnostics DiaSpect Tm Hemoglobin Analyzer
- HemoCue Hb 201+ Analyzer
- HemoCue Hb 301 System.
- HemoCue Hb 801 System
- HemoCue Plasma/Low Hb System
- Abbott m-PIMA ANALYSER
- SD Biosensors STANDARD LipidoCare Analyzer
- Abbott AFINION 2 Analyzer
- Abbott DIGIVAL
- Abbott ID NOW
- Abbott NYCOCARD READER II
- Siemens DCA Vantage Analyzer
- Abbott UROMETER 720 URINE CHEMISTRY ANALYZER
- Acon Mission U120 Urine Analyzer
- Acon Mission U500 Urine Analyzer
- Arkray Compact Urine Analyzer PocketChem UA PU-4010
- Siemens CLINITEK Status+ Analyzer
- Urit 31 Urine Analyzer
- HemoCue WBC DIFF System
- HemoCue Safety Lancets
- Roche Accu-Chek Accu-Fine insulin pen needles
- Roche Accu-Chek Softclix Lancet
- HemoCue Albumin 201 Microcuvettes (50T)
- Arkray PocketChem Blood Ammonia Strips (50T)
- FUJIFILM DRI-CHEM SLIDE NH3-WII (for whole blood) and NH3-PII (for plasma)
- Wondfo Blood Gas Analyzer Test Card
- Abbott PIMA CD4 CARTRIDGE (100T)
- Abbott PIMA CD4 CARTRIDGE (25T)
- Abbott CHOLESTECH LDX LIPID PROFILE CASSETTES
- Abbott CHOLESTECH LDX TC CASSETTES
- Abbott CHOLESTECH LDX TC GLU CASSETTES
- Abbott CHOLESTECH LDX TC HDL CASSETTES
- Abbott CHOLESTECH LDX TC HDL GLU CASSETTES
- Acon Mission Cholesterol Test Devices- 3-in-1 Lipid Panel
- Acon Mission Ultra Cholesterol Test Strips
- Roche CoaguChek XS PT Test Strips (24T)
- Roche CoaguChek XS PT Test Strips (48T)
- Arkray SPOTCHEM E- Plate
- Acon On Call Plus Blood Glucose Test Strips (200T)
- Acon On Call Plus Blood Glucose Test Strips (50T)
- Arkray GLUCOCARD G-1070 Test Strips
- Arkray GLUCOCARD Vital GT-1050
- HemoCue Glucose 201 RT Microcuvettes
- Roche Accu-Chek Guide Test Strips (50T)
- Roche Accu-Chek Active Test Strips (100T)
- Accu-Chek Active Test Strips (50T)
- Roche Accu-Chek Instant Test Strips (25T)
- SD Biosensor SD CodeFree Blood Glucose Test Strip
- Arkray PocketChem A1c HbA1c Test Kit
- EKF Diagnostics Quo-Test A1c Test Cartridge Kit
- HemoCue HbA1c 501 Test Cartridge
- Acon Mission Hb Hemoglobin Test Strips
- Mission HemoPro Hemoglobin Microcuvette
- Arkray Pocketchem HemoG Test Strips
- Boditech Hemochroma PLUS Microcuvette
- COPACK GMBH Heamoglobin Color Scale.
- EKF Diagnostics Hemo Control Microcuvette
- EKF Diagnostics Hemo_Control Control Cuvettes
- HemoCue Hb 201 Control Set
- HemoCue Hb 801 Microcuvettes
- HemoCue Hb301 Control Set 3
- HemoCue Plasma Low Hb Control
- Abbott m-PIMA HIV-1/2 DETECT
- Abbott m-PIMA HIV-1/2 VIRAL LOAD TEST
- SD Biosensor STANDARD LipidoCare Lipid Test Strip - Lipid Profile
- Abbott AFINION HbA1c
- Abbott AFINION LIPID PANEL
- Abbott i-STAT ACTc Cartridge
- Abbott i-STAT ACTk Cartridge
- Abbott i-STAT BNP CARTRIDGE
- Abbott i-STAT CG4+ Cartridge
- Abbott i-STAT CG8+ Cartridge
- Abbott i-STAT CHEM8+ CARTRIDGE
- Abbott i-STAT CK-MB CARTRIDGE
- Abbott i-STAT EC8+ Cartridge
- Abbott i-STAT PT/INR CARTRIDGE
- i-STAT Total beta-hCG Cartridge
- Abbott ID NOW INFLUENZA A & B 2
- ID NOW RSV
- Abbott ID NOW STREP A 2
- Abbott NYCOCARD CRP
- Abbott NYCOCARD HbA1c
- Siemens DCA HbA1c Reagent Kit for DCA Vantage Analyzer
- Siemens DCA Microalbumin/Creatinine Urine Test
- Abbott UroColor 2 (Protein, Glucose)
- Abbott UroColor 2K (Ketone,Glucose)
- Abbott UroColor 4
- Abbott UroColor 4S
- Acon Mission Liquid Urine Control
- Acon Mission Urinalysis Strips - 10P (100T)
- Acon Mission Urinalysis Strips - 14P (25T)
- Acon Mission Urinalysis Strips - 2P (Creatinine & Albumin)
- Acon Mission Urinalysis Strips - 4P
- Arkray Urine test strips AUTION SCREEN
- Arkray Urine test strips AUTION Sticks
- Multistix 10 SG Reagent Strips
- Siemens Uristix Reagent Urinalysis Strips
- URIT 10G Urine Reagent Strips
- Roche CoaguChek Pro II
- Roche cobas pulse system
- Roche Cobas h 232 POC system
- Roche Urisys 1100 urine analyzer
- Abbott i-STAT 6+ CARTRIDGE
- Roche Coagu Chek Pro II
-
Medical Analyzer
- Mindray Clinical Chemistry Analyzer BS-450
- Snibe Maglumi 600 Chemiluminescence Immunoassay (CLIA) System
- Eppendorf PCR Tubes
- Erba Mannheim EC 90 Next Generation Electrolyte Analyzer
- Erba Mannheim EM Destiny 180 Fully Automated Clinical Chemistry Analyzer
- Erba Mannheim LIQUIXX Creatinine Kinase Kit
- BioRad QX600 Droplet Digital PCR System
- Abbott Alere h s 30-3-part differential hematology Analyzer
- Awareness Technology ChemWell-T 4620- PC Controlled Chemistry + Turbidmetric Analyzer
- Thermo Fisher Scientific Wellwash Microplate Washer
- Snibe Maglumi 2000 Plus Chemiluminescence Immunoassay (CLIA) System
- Roche Cobas U 601 Urine Analyzer
- Qiagen qPCR 384-well plate white, skirted
- Qiagen Filter-Tips, 1000 l
- Qiagen qPCR 96-well plate, white, skirted
- Snibe MAGLUMI X3 Fully-auto Chemiluminescence Immunoassay (CLIA) System
- Qiagen QIAxcel Connect
- Snibe Maglumi 800 Chemiluminescence Immunoassay (CLIA) System
- Snibe MAGLUMI X8 Fully-auto Chemiluminescence Immunoassay (CLIA) System
- Medica EasyLyte Analyzer
- Thermo Fisher Scientific QuantStudio 1 Real-Time PCR System
- Mindray Estradiol (CLIA)
- Awareness Technology ChemWell 2902- PC Controlled Chemistry Analyzer
- Erba Mannheim ECL 412 Four Channel Semi Automated Coagulation Analyzer
- Thermo Fisher Scientific KingFisher Flex Purification System
- Thermo Fisher Scientific Applied Biosystems MagMAX Viral/Pathogen Ultra Nucleic Acid Isolation Kit
- Erba Mannheim H560 Automated 5-Part differential hematology Analyzer
- Awareness Technology ChemWell Fusion- 4800 PC Controlled EIA and CLIA Combination Analyzer
- Thermo Fisher Scientific 7500 Fast Real-Time PCR System
- Snibe MAGLUMI X6 Fully-auto Chemiluminescence Immunoassay (CLIA) System
- Abbott Alere h 380-3-part differential hematology Analyzer
- Philips SimplyGo Portable oxygen concentrator
- Abbott Alere Dip-5P
- QuantStudio 5 Real Time PCR System 96-well
- Leica Biosystems Microtomes
- Awareness Technology ChemWell-T 4625 ics- PC Controlled Chemistry + Turbidmetric Analyzer
- Qiagen 8-Rod Covers
- Qiagen QIAcuity Nanoplate 26k 24-well
- Roche Cobas 4000 C311 Stand Alone System
- Eppendorf twin.tec Trace PCR Plates BioBased PCR Clean
- Thermo Fisher Scientific Multiskan FC Microplate Photometer
- Erba Mannheim EC90 Urine Diluent
- Roche Cobas 6000 e601 Module System
- Qiagen QIAcuity One, 5plex System
- Qiagen QIAprep 96 Plates (24)
- Qiagen EasiCollect Plus (50)
- Siemens RAPIDChem 744 Na+K+Cl- Reagent Module
- Qiagen Nanoplate Tray
- Mindray Clinical Chemistry Analyzer BS-230
- Qiagen QIAcube Connect
- Thermo Fisher Scientific Invitrogen MagMAX-96 Blood RNA Isolation Kit
- Qiagen QIAcube HT Plasticware
- Mindray Auto Hematology Analyzer BC-6800Plus
- Qiagen Elution Microtubes CL
- Philips EverFlo 5 LPM Oxygen Concentrator
- Quantstudio 3 Real Time Pcr System 0.2 mL 96 wells
- T100 Thermal Cycler
- Applied Biosystems SimpliAmp and MiniAmp Thermal Cycler
- Truelab Quattro Real Time Quantitative Micro PCR Analyzer
- Spincell 5 Compact Hematology Analyzer
- Automated Thermal Cycler (ATC) 384 Well Laptop 1-m Cable
- Thermofisher Quantstudio 12k Flex Real Time PCR System 0.2 mL 96 wells
- Qiagen Rotor GENE Q PCR Machine
- QIAquant 96 Real time PCR System
- Applied Biosystems Veriti Pro 96 Well Thermal Cycler
- Applied Biosystems QuantStudio 7 Flex Real Time PCR System
- 7500 Thermo Fisher Fast Real Time PCR System
- LightCycler 96 Real-Time PCR System
- Applied Biosystem ProFlex 3 x 32-well PCR System
- Philips SimplyGo Mini Portable Oxygen Concentrator
- CFX Opus 96 Real-Time PCR Instrument 0.2 mL 96 Wells
- BioRad CFX 96well Touch RTPCR System
- Eppendorf Refrigerated Centrifuge 5425 R
- Eppendorf CellXpert C170i CO2 Incubators
- Philips Trilogy EV300 Ventilator
- Fully Automatic Semi-Auto Chemistry and Cogulation Analyser
- Eppendorf Biospectrometer Basic
- ALTA Nucleic Acid extractor
- Forma Steri-Cult CO2 Incubators
- Sysmex XN-350/ XN-550 XN-L Series Hematology
- KingFisher Flex Purification System King Fisher with 96 PCR Head.
- Invitrogen Countess 3 Automated Cell Counter
- Applied Biosystems QuantStudio 1 Real Time PCR System 96well 0.2 mL
- Biorad C1000 Touch Thermal Cycler with 96-Well Fast Reaction Module 1851196
- SimpliAmp Thermal Cycler
- Awareness Technology ChemWell RPR 5801
- Awareness Technology ChemWell 2910- PC Controlled ELISA/ BIOCHEMISTRY ANALYZER
- Awareness Technology Stat Fax 1904-Semi Automatic Chemistry Analyzer
- Awareness Technology Stat Fax 3300- Semi Automatic Chemistry Analyzer
- Awareness Technology Stat Fax 4500- Semi Automatic Chemistry Analyzer
- Erba Mannheim CHEM 5X Clinical Chemistry Analyzer
- Erba Mannheim CHEM 6 Semi-automated Clinical Chemistry Analyzer
- Erba Mannheim CHEM 7 First class semi automated clinical chemistry Analyzer
- Erba Mannheim CHEM Touch Semi Automated Clinical Chemistry Analyzer
- Erba Mannheim EM 200 Fully Automated Random Access Clinical Chemistry Analyzer
- Erba Mannheim EM 360 Fully Automated Clinical Chemistry Analyzer
- Erba Mannheim XL 200 Fully Automated Clinical Chemistry Analyzer
- Erba Mannheim XL 640 CRS Fully Automated Clinical Chemistry Analyzer with Autoloader
- Erba Mannheim XL 640 Fully Automated Clinical Chemistry Analyzer
- FUJIFILM DRI-CHEM NX600
- FUJIFILM DRI-CHEM NX700
- Medica EasyRA Clinical Chemistry Analyzer
- Roche Cobas e 411 Analyzer
- Quidel's Triage System
- Erba Mannheim ECL 105 Single Channel Cogulation Analyzer
- Erba Mannheim ECL 760 Seven Channel Fully Automated Random Access Analyzer
- Siemens BFT II Analyzer
- Sysmex CA-104 Coagulation Analyzer
- Sysmex CA-660 Automated Haemostasis Analyzer
- Wondfo Optical Coagulation Analyzer
- Awareness Technology Selectalyte 3910- ISE Analyzer
- Roche 9180 Electrolyte Analyzer
- Siemens RAPIDChem 744/754 Electrolyte and Lithium Testing Analyzers
- Acon Mission HA-360 3-diff Automatic Hematology Analyzer
- Arkray Automatic Glycohemoglobin Analyzer ADAMS A1c HA-8180V
- BioRad D-10 Hemoglobin Testing System
- BioRad VARIANT II TURBO Hemoglobin Testing System
- Erba Mannheim Cube 30 Touch Automated ESR Analyzer
- Erba Mannheim ELITE 580 Advance Hematology Analyzer
- Erba Mannheim ESL 30 Fully Automated walk-away System
- Erba Mannheim H360 Automated 3-Part differential hematology Analyzer
- Erba Mannheim Hb-820 Automated Hba1c Testing System
- Minicube ESR Analyzer
- Medica EasyBloodGas Analyzer
- Medica Medicas EasyStat Blood gas Analyzer
- Sysmex XN-1000 Hematology Analyzer
- Sysmex XN-330 Automated Hematology Analyzer
- Sysmex XN-350 Automated Hematology Analyzer
- Sysmex XN-550 Automated Hematology Analyzer
- Sysmex XP-300 Automated Hematology Analyzer
- Sysmex XQ-320 Automated Hematology Analyzer
- Leica BIOSYSTEM HistoCore AUTOCUT
- Leica BIOSYSTEM HistoCore BIOCUT
- Leica BIOSYSTEM HistoCore MULTICUT
- Abbott Alere Easy Microplate Washer
- Abbott Alere Easy Reader
- Awareness Technology ChemWell 2 5100 PC Controlled EIA Analyzer
- Awareness Technology Chromate 4300- PC Controlled Microplate Washer
- Awareness Technology LuMate 4400-PC Controlled Microplate Luminometer
- Awareness Technology Stat Fax 2200- Digital Dry Bath
- Awareness Technology Stat Fax 2600- Automatic Microplate Washer
- Awareness Technology Stat Fax 4200 - Stand-alone Microplate Reader
- Awareness Technology Stat Fax 4700 - Micro strip Reader
- ELAN 30s Fully Automated ELISA Microstrip Processor
- Erba Mannheim Lisa Wash II
- Erba Mannheim LisaScan EM
- Thermo Fisher Scientific Varioskan LUX multimode microplate reader
- Wondfo Accre 8 Automatic Chemiluminescence Immunoassay Analyzer
- BioRad C1000 Touch Thermal Cycler with 384-Well Reaction Module
- BioRad C1000 Touch Thermal Cycler with 96-Deep Well Reaction Module
- BioRad C1000 Touch Thermal Cycler with 96-Well Fast Reaction Module
- BioRad CFX Duet Real-Time PCR System
- BioRad CFX Opus 384 Real-Time PCR System
- BioRad CFX Opus 96 Real-Time PCR System
- BioRad CFX Opus Deepwell Real-Time PCR System
- BioRad CFX96 Touch Real-Time PCR Detection System
- BioRad PTC Tempo 96 Thermal Cycler
- BioRad PX1 PCR Plate Sealer
- BioRad S1000 Thermal Cycler with 96-Well Fast Reaction Module
- BioRad T100 Thermal Cycler
- BioRad TC20 Automated Cell Counter
- Cepheid GeneXpert II 2 Module Instrument
- Cepheid GeneXpert Infinity-48s
- Cepheid GeneXpert IV 4 Module Instrument
- Cepheid GeneXpertn XVI 16 Module Instrument
- Eppendorf epMotion 5073t NGS Solution
- Eppendorf Mastercycler nexus - PCR Thermal Cycler
- Eppendorf Mastercycler nexus GX2 - PCR Thermal Cycler
- Eppendorf Mastercycler nexus X2 - PCR Thermal Cycler
- Eppendorf Mastercycler X40 - PCR Thermocycler
- Eppendorf Mastercycler X50 - PCR Thermocycler
- Qiagen QIAquant 96 5 Plex Real-Time PCR Thermal Cycler
- Qiagen Rotor-Gene Q 5plex HRM System
- Roche LightCycler 480 System
- Roche LightCycler 96 System
- Thermo Fisher Scientific 7500 Fast Dx Real-Time PCR System
- Thermo Fisher Scientific Automated Thermal Cycler 384-well (ATC)
- Thermo Fisher Scientific Countess 3 Automated Cell Counter
- Thermo Fisher Scientific MiniAmp Plus Thermal Cycler
- Thermo Fisher Scientific MiniAmp Thermal Cycler
- Thermo Fisher Scientific ProFlex 3 x 32-well PCR System
- Thermo Fisher Scientific ProFlex 96-well PCR System
- Thermo Fisher Scientific QuantStudio 12K Flex Real-Time PCR System
- Thermo Fisher Scientific QuantStudio 3 Real-Time PCR System
- Thermo Fisher Scientific QuantStudio 5 Real-Time PCR System ( 384 Wells )
- Thermo Fisher Scientific QuantStudio 5 Real-Time PCR System ( 96 Wells )
- Thermo Fisher Scientific QuantStudio 6 Pro Real-Time PCR System
- Thermo Fisher Scientific QuantStudio 7 Flex Real-Time PCR System
- Thermo Fisher Scientific QuantStudio 7 Pro Real-Time PCR System
- Thermo Fisher Scientific SeqStudio 8 Flex Genetic Analyzer
- Thermo Fisher Scientific SimpliAmp Thermal Cycler
- Thermo Fisher Scientific VeritiPro Thermal Cycler
- Philips EverFlo Home oxygen system
- Philips SimplyGo Mini Portable oxygen concentrator (POC)
- Philips Trilogy EV300 Hospital ventilator
- Erba Mannheim LAURA Urine Analyzer
- Erba Mannheim Urine analyzer LAURA Smart
- Awareness Technology DriDye Check Strips
- Awareness Technology RediCheck Test Kit
- Erba Mannheim Erba Control N
- Erba Mannheim Erba Control P
- FUJIFILM FUJI DRI-CHEM CALIBRATOR CP (CRP)
- Roche Cobas b 221 system
- Roche cobas c 111 Chemistry analyzer
- Thermo Fisher Scientific Applied Biosystems GlobalFiler PCR Amplification Kit
- Arkray Automatic Glycohemoglobin Analyzer ADAMS A1c Lite HA-8380V
- Arkray COBIO S50
- Erba Microalbumin Kit
- Mindray Clinical Chemistry Analyzer BS-240
- Mindray Chemistry Analyzer BS-240Pro
- Mindray Chemistry Analyzer BS-430
- Mindray Chemistry Analyzer BS-480
- Mindray Auto Hematology Analyzer BC-20s
- Mindray Auto Hematology Analyzer BC-5150
- Mindray Auto Hematology Analyzer BC-6000
- Mindray Auto Hematology Analyzer BC-6200
- Mindray Chemiluminescence Immunoassay System CL-900i
- Mindray Chemiluminescence Immunoassay System CL-1000i
-
Medical Reagents
- FUJIFILM FUJI DRI-CHEM SLIDE CRE-P III
- Acon Cyto-Diluent for Mission HA-360
- Qiagen QIAcuity OneStep Advanced Probe Kit (1 ml)
- Snibe MAGLUMI ACTH (CLIA)
- Snibe MAGLUMI PG II (CLIA)
- Snibe MAGLUMI PIIIP N-P (CLIA)
- Thermo Fisher Scientific Applied Biosystems TaqPath ProAmp Master Mix
- FUJIFILM FUJI DRI-CHEM SLIDE DBIL-P II
- Erba Mannheim LIQUIXX LDH-P Kit
- Erba Mannheim System Pack NORM Kit
- FUJIFILM FUJI DRI-CHEM SLIDE GLU-P III
- FUJIFILM FUJI DRI-CHEM SLIDE CA-P III
- Mindray Cholinesterase (C&Q) Reagent Kit
- Erba Mannheim LIQUIXX Calcium Kit
- FUJIFILM FUJI DRI-CHEM SLIDE AMYL-P III
- Erba Mannheim System Pack Amylase Kit
- Erba Mannheim System Pack Bilirubin Total Kit
- Mindray Prolactin (CLIA)
- Mindray LDL-C Reagent Kits
- Qiagen RT SYBR Green Fluor qPCR Mastermix (24)
- Qiagen Ni-NTA Superflow
- Qiagenq PCR 384-well plate white, skirted
- Qiagen QIAquick 96 PCR Purification Kit (24)
- Qiagen QuantiNova Probe PCR Kit
- Qiagen QIAfilter Plasmid Midi Kit (100)
- Qiagen Microbial DNA qPCR Assay Kit
- Qiagen dPCR CNV Probe Reference Assay
- Qiagen Puregene Blood Kit (1000 ml)
- Qiagen QIAamp DNA Mini QIAcube Kit (240)
- Qiagen QIAamp MinElute ccfDNA Midi Kit (50)
- Qiagen QIAcuity EG PCR Kit (1 ml)
- QIAGEN Plasmid Mega Kit (25)
- Mindray Folate (CLIA)
- Mindray Free Thyroxine (CLIA)
- Mindray Thyroid-Stimulating Hormone (CLIA)
- Mindray Cancer Antigen 125 (CLIA)
- Qiagen RNeasy 96 Universal Tissue Kit (4)
- Snibe MAGLUMI PRL (CLIA)
- Snibe MAGLUMI CA 19-9 (CLIA)
- Qiagen QuantiTect Rev. Transcription Kit (50)
- Erba Mannheim System Pack Magnesium Kit
- Qiagen REPLI-g Advanced DNA Single Cell Kit (96)
- Qiagen REPLI-g Cell WGA & WTA Kit (48)
- Qiagen REPLI-g Single Cell Kit (96)
- FUJIFILM FUJI DRI-CHEM SLIDE TBIL-P III
- Mindray Parathyroid hormone (CLIA)
- Qiagen miRCURY LNA miRNA PCR Assay
- Qiagen Generation DNA Purif. Solution (1000 ml)
- Snibe MAGLUMI Starter 1+2
- Mindray Bilirubin Direct Reagent Kits
- Qiagen QuantiFast Multiplex PCR Kit (400)
- Qiagen EZ1&2 DNA FFPE Kit (48)
- Erba Mannheim ERBA Lipase Eco Kit
- Snibe MAGLUMI AFP (CLIA)
- Snibe MAGLUMI Proinsulin (CLIA)
- Snibe MAGLUMI TSH (CLIA)
- Thermo Fisher Scientific Invitrogen Quant-iT Protein Assay Kit
- Erba Mannheim System Pack Iron - FE 125 Kit
- Mindray M-6 LN Lyse Hematology Reagent
- Qiagen QuantiTect Primer Assay (200)
- Qiagen QIAseq Human Exome Kit (96)
- Qiagen QIAsymphony Virus/Bacteria Mini Kit (192)
- Thermo Fisher Scientific Invitrogen Platinum SuperFi II PCR Master Mix
- Qiagen EpiTect MethyLight PCR +ROX Vial Kit (200)
- Erba Mannheim System Pack Uric Acid Kit
- Qiagen EpiTect Hi-C Kit (6)
- Qiagen EpiTect 96 Bisulfite Kit (2)
- Qiagen DNeasy mericon 96 QIAcube HT Kit (5)
- Qiagen dNTP Set, PCR Grade
- Qiagen QIAamp 96 PowerFecal QIAcube HT Kit (5)
- Thermo Fisher Scientific Applied Biosystems MicroAmp Fast Optical 96-Well Reaction Plate, 0.1 mL
- Erba Mannheim LIQUIXX Phosphorous Kit
- MIndray Carbohydrate Antigen 19-9 (CLIA)
- Qiagen QuantiFast Pathogen RT-PCR +IC Kit (400)
- Erba Mannheim System Pack Total Protein Kit
- Qiagen dPCR CGT Assay GFP (FAM)
- FUJIFILM FUJI DRI-CHEM SLIDE TP-P III
- Qiagen dPCR Copy Number Assay (200)
- Qiagen Cell and Gene Therapy Viral Vector Lysis Kit (100 rxn)
- Qiagen dPCR LNA Mutation Assay (200)
- Qiagen DNeasy 96 Blood & Tissue Kit (12)
- Qiagen dPCR Microbial DNA Detection Assays
- Qiagen AllTaq PCR Core Kit (5000 U)
- Erba Mannheim LIQUIXX Creatinine Kit
- Erba Mannheim System Pack Ferritin Kit
- ARKRAY Albumin Liquid Reagent
- Siemens Stratus CS Acute Care Troponin I TestPak
- Qiagen AllPrep DNA/RNA FFPE Kit (50)
- Thermo Fisher Scientific Invitrogen PureLink Pro 96 total RNA Purification Kit
- Qiagen EZ1&2 DNA Investigator Kit (48)
- Qiagen exoRNeasy Maxi Kit (50)
- Qiagen DyeEx 96 Kit (24)
- Qiagen HotStarTaq DNA Polymerase (25000)
- Qiagen RNeasy 96 Kit (12)
- Qiagen Type-it Fast SNP Probe PCR Kit (800)
- Qiagen REPLI-g Screening Kit (200)
- Qiagen QuantiNova LNA PCR Assay (750)
- Qiagen TAGZyme DAPase Enzyme (50 U)
- Qiagen RNeasy 96 QIAcube HT Kit (5)
- Qiagen TurboCapture 96 mRNA Kit (5)
- Qiagen RNeasy Mini QIAcube Kit (240)
- Qiagen RNeasy Protect Mini Kit (250)
- Qiagen Taq DNA Polymerase (25000)
- Mindray Total Prostate Specific Antigen (CLIA)
- Qiagen RNeasy UCP Micro Kit
- Qiagen RT SYBR Green ROX qPCR Mastermix (24)
- Mindray Total Thyroxine (CLIA)
- Qiagen QIAwave RNA Plus Mini Kit (250)
- Qiagen QIAwave RNA Mini Kit (250)
- Qiagen RT2 Profiler PCR Array
- Mindray Testosterone (CLIA)
- Qiagen QuantiTect Virus Kit (200)
- Mindray Total Human Chorionic Gonadotropin (CLIA)
- Qiagen QIAseq FastSelect Epidemiology Kit (96)
- Qiagen QIAseq FX DNA Library Kit HT A-D (384)
- Mindray Procalcitonin (CLIA)
- Qiagen REPLI g Midi Kit (100)
- Qiagen QIAseq cfDNA All-in-One Kit (96)
- Qiagen QIAamp 96 Virus QIAcube HT Kit (5)
- Qiagen QIAamp DSP DNA Mini Kit (50)
- Qiagen QIAamp ccfDNA/RNA Kit (50)
- Qiagen QIAprep 96 Turbo Core Kit (24)
- Mindray Progesterone (CLIA)
- Qiagen QIAamp Viral RNA Mini QIAcube Kit (240)
- Qiagen QIAcuity Probe PCR Kit (5 mL)
- Mindray Neuron-specific enolase (CLIA)
- Mindray Total Triiodothyronine (CLIA)
- Qiagen QIAamp DNA Blood Maxi Kit (50)
- Qiagen QIAprep& Viral RNA UM Kit (2400)
- Qiagen QIAamp DNA Micro Kit (50)
- QIAGEN Plasmid Plus Maxi Kit (100)
- Qiagen QIAamp UltraSens Virus Kit (250)
- Qiagen QIAamp DNA Blood Midi Kit (100)
- Qiagen QIAamp 96 DNA QIAcube HT Kit (5)
- Mindray Troponin I (CLIA)
- Mindray Carcinoembryonic Antigen (CLIA)
- Mindray Follicle Stimulating Hormone (CLIA)
- Mindray Estriol (CLIA)
- Mindray Free Prostate Specific Antigen (CLIA)
- Mindray Ferritin (CLIA)
- Mindray Free Triiodothyronine (CLIA)
- Mindray Luteinizing Hormone (CLIA)
- Mindray Myoglobin (CLIA)
- Mindray CYFRA 21-1 (CLIA)
- Qiagen Ni-NTA Agarose (500 ml)
- Mindray Phosphorus Reagent Kits
- Mindray Dehyro-epi-androsterone sulfate (CLIA)
- Mindray Insulin (CLIA)
- Qiagen QIAamp 96 Viral RNA Kit (10)
- Mindray Cortisol (CLIA)
- Mindray Creatine kinase MB (CLIA)
- Mindray Uric Acid Reagent Kits
- Mindray Lactate Dehydrogenase Reagent Kits
- Mindray C-peptide (CLIA)
- Mindray Total Protein Reagent Kits
- Mindray Anti-mullerian Hormone (CLIA)
- Mindray Magnesium Reagent Kits
- Mindray Alpha-fetoprotein (CLIA)
- Mindray Cancer Antigen 72-4 (CLIA)
- Mindray Cancer Antigen 15-3 (CLIA)
- Mindray B-type natriuretic peptide (CLIA)
- Mindray UIBC (C) Reagent Kits
- Mindray Microalbumin (C) Reagent Kits
- Mindray Glucose (Hex) Reagent Kits
- Mindray Bilirubin Total (DSA) Reagent Kits
- Mindray T P in Urine/ CFS Reagent Kits
- Mindray Antibody to thyroid peroxidase (CLIA)
- Mindray Calcium Reagent Kits
- Qiagen Puregene Tissue Kit (33 g)
- Qiagen miRCURY LNA SYBR Green PCR Kit
- Mindray GGT Reagent Kits
- Mindray 25-OH-Vitamin D Total (CLIA)
- Mindray Bilirubin Total Reagent Kits
- Mindray Adenosine deaminase Reagent Kits
- BioRad ReadyPrep Protein Extraction Kit (Total Protein)
- Qiagen PowerProtect DNA/RNA (1000 ml)
- Qiagen QIAamp Circulating Nucleic Acid Kit (50)
- Thermo Fisher Scientific Invitrogen PureLink PCR Micro Kit
- Snibe MAGLUMI TPA (CLIA)
- Snibe MAGLUMI Total PSA (CLIA)
- Qiagen QIAamp 96 DNA Blood Kit (12)
- Qiagen MinElute 96 UF PCR Purification Kit (24)
- Qiagen FlexiGene DNA Kit (250)
- QIAGEN Plasmid Plus 96 Miniprep Kit (4)
- Snibe MAGLUMI TG (CLIA)
- Snibe MAGLUMI T3 (CLIA)
- Snibe MAGLUMI SCCA (CLIA)
- Snibe MAGLUMI S-100 (CLIA)
- Qiagen EpiTect Fast 96 FFPE Bisulfite Kit
- Qiagen Hot StarTaq Master Mix Kit (2500 U)
- Snibe MAGLUMI PRG (CLIA)
- Erba Mannheim LIQUIXX Urea (BUN) Kit
- Snibe MAGLUMI CA 125 (CLIA)
- Thermo Fisher Scientific Invitrogen PureLink DNase Set
- Mindray M-6FN DYE Hematology Reagent
- Thermo Fisher Scientific Invitrogen PureLink RNA Micro Scale Kit
- Thermo Fisher Scientific Invitrogen Quant-iT RNA Assay Kits and Quant-iT RNA HS Reagent
- Mindray Vitamin B12 (CLIA)
- Thermo Fisher Scientific Invitrogen PureLink Total RNA Blood Kit
- Thermo Fisher Scientific Invitrogen SuperScript One-Step RT-PCR System with Platinum Taq DNA Polymerase
- Thermo Fisher Scientific Invitrogen PureLink RNA Mini Kit
- Snibe MAGLUMI S-Troponin I (CLIA)
- Thermo Fisher Scientific Invitrogen Quant-iT dsDNA Assay Kits, high sensitivity (HS) and broad range (BR)
- Snibe MAGLUMI TMA (CLIA)
- Snibe MAGLUMI PCT (CLIA)
- Snibe MAGLUMI TGA (CLIA)
- Snibe MAGLUMI Estradiol (CLIA)
- Snibe MAGLUMI Vitamin B12 (CLIA)
- Snibe MAGLUMI Rev T3 (CLIA)
- Snibe MAGLUMI IAA (CLIA)
- Snibe MAGLUMI beta2-MG (CLIA)
- Snibe MAGLUMI FT3 (CLIA)
- Snibe MAGLUMI IgE (CLIA)
- Snibe MAGLUMI Albumin (CLIA)
- Snibe MAGLUMI PG I (CLIA)
- Snibe MAGLUMI TRAb (CLIA)
- Snibe MAGLUMI Testosterone (CLIA)
- Snibe MAGLUMI T4 (CLIA)
- Snibe MAGLUMI Aldosterone (CLIA)
- Snibe MAGLUMI HA (CLIA)
- Qiagen MagAttract Magnetic Rack
- Mindray Thyroglobulin (CLIA)
- FUJIFILM FUJI DRI-CHEM SLIDE AUTO TIPS
- Mindray Calcitonin (CLIA)
- Qiagen Type-it HRM PCR Kit (400)
- Qiagen REPLI-g WTA Single Cell Kit (96)
- Erba Mannheim EC Cartridge M (1000T)
- FUJIFILM FUJI DRI-CHEM SLIDE IP-P
- FUJIFILM FUJI DRI-CHEM REFERENCE FLUID RE
- Wondfo Thrombin Time (TT)
- BioRad SsoFast EvaGreen Supermix, 200 x 20 l rxns, 2 ml (2 x 1 ml)
- BioRad QX ONE Droplet Digital PCR (ddPCR) System
- ARKRAY TURBIGOLD CRP
- Cepheid Xpert HPV
- Cepheid Xpert HIV-1 Viral Load
- FUJIFILM FUJI DRI-CHEM SLIDE GOT/AST-P III
- Thermo Fisher Scientific Invitrogen RNaseZap RNase Decontamination Solution
- Snibe MAGLUMI 25-OH Vitamin D (CLIA)
- FUJIFILM FUJI DRI-CHEM SLIDE Na-K-Cl
- Erba Mannheim ERBA Auto Wash Kit
- Erba Mannheim ERBA Microprotein Reagent Kit
- Erba Mannheim ERBA Wash Kit
- Erba Mannheim Erba Protime LS
- Erba Mannheim LIQUIXX Albumin Kit
- Erba Mannheim LIQUIXX Alkaline Phosphates Kit
- Erba Mannheim LIQUIXX Amylase Kit
- Erba Mannheim LIQUIXX Bilirubin Total & Direct
- Erba Mannheim LIQUIXX Cholesterol Kit
- Erba Mannheim LIQUIXX CK-MB Kit
- Erba Mannheim LIQUIXX Creatinine Enzymatic Kit
- Erba Mannheim LIQUIXX Gamma GT Kit
- Erba Mannheim LIQUIXX Glucose Kit
- Erba Mannheim LIQUIXX HbA1c with Calibrator
- Erba Mannheim LIQUIXX HDL-Cholesterol Kit
- Erba Mannheim LIQUIXX HDL-Direct Kit
- Erba Mannheim LIQUIXX SGOT-R Kit
- Erba Mannheim LIQUIXX SGPT-R Kit
- Erba Mannheim LIQUIXX T-LTX ASO Kit
- Erba Mannheim LIQUIXX T-LTX CRP Kit
- Erba Mannheim LIQUIXX T-LTX RF Kit
- Erba Mannheim LIQUIXX Total Protein Kit
- Erba Mannheim LIQUIXX Triglycerides Kit
- Erba Mannheim LIQUIXX-M Triglycerides Kit
- Erba Mannheim LIQUIXX-M Uric Acid Kit
- Erba Mannheim System Pack Albumin Kit
- Erba Mannheim System Pack Alkaline Phosphatase Kit
- Erba Mannheim System Pack Bilirubin Direct Kit
- Erba Mannheim System Pack C-Reactive Protein (CRP)
- Erba Mannheim System Pack Calcium Kit
- Erba Mannheim System Pack Chloride Kit
- Erba Mannheim System Pack Cholesterol Kit
- Erba Mannheim System Pack CK NAC
- Erba Mannheim System Pack CK-MB Kit
- Erba Mannheim System Pack Creatinine Kit
- Erba Mannheim System Pack Gama Glutamyl transferase (GGT)
- Erba Mannheim System Pack Glucose Kit
- Erba Mannheim System Pack HbA1c cali. Set
- Erba Mannheim System Pack HDL Cholesterol Kit
- Erba Mannheim System Pack Lipase Kit
- Erba Mannheim System Pack Path Kit
- Erba Mannheim System Pack Phosphorus Kit
- Erba Mannheim System Pack SGOT Kit
- Erba Mannheim System Pack SGPT Kit
- Erba Mannheim System Pack Triglycerides
- Erba Mannheim System Pack UIBC 125
- Erba Mannheim System Pack Urea Kit
- Erba Mannheim System Pack XL Multical Kit
- FUJIFILM FUJI DRI-CHEM SLIDE ALB-P
- FUJIFILM FUJI DRI-CHEM SLIDE ALP-P III
- FUJIFILM FUJI DRI-CHEM SLIDE BUN-P III
- FUJIFILM FUJI DRI-CHEM SLIDE CHE-P
- FUJIFILM FUJI DRI-CHEM SLIDE CKMB-P
- FUJIFILM FUJI DRI-CHEM SLIDE CPK-P III
- FUJIFILM FUJI DRI-CHEM SLIDE CRP-S III
- FUJIFILM FUJI DRI-CHEM SLIDE GPT/ALT-P III
- FUJIFILM FUJI DRI-CHEM SLIDE HDL-C-P III D
- FUJIFILM FUJI DRI-CHEM SLIDE LAP-P
- FUJIFILM FUJI DRI-CHEM SLIDE LDH-P III
- FUJIFILM FUJI DRI-CHEM SLIDE LIP-P
- FUJIFILM FUJI DRI-CHEM SLIDE Mg-P III
- FUJIFILM FUJI DRI-CHEM SLIDE NH3-P II
- FUJIFILM FUJI DRI-CHEM SLIDE TCHO-P III
- FUJIFILM FUJI DRI-CHEM SLIDE TCO2-P
- FUJIFILM FUJI DRI-CHEM SLIDE TG-P III
- FUJIFILM FUJI DRI-CHEM SLIDE UA-P III
- Wondfo Activated Clotting Time (ACT)
- Wondfo Activated Partial Thromboplastin (APTT)
- Wondfo Fibrinogen Reagent Kit (Clotting)
- Wondfo Prothrombin Time (PT)
- Siemens RAPIDChem 744 Daily Cleaner Kit
- Acon Cyto-Lyser for Mission HA-360
- Acon Mi-Po Cleaner for Mission HA-360
- Cepheid Xpert BCR-ABL Ultra
- Cepheid Xpert C. difficile
- Cepheid Xpert Carba-R
- Cepheid Xpert CT/NG
- Cepheid Xpert EV
- Cepheid Xpert FII & FV
- Cepheid Xpert GBS
- Cepheid Xpert HBV Viral Load
- Cepheid Xpert HCV Viral Load
- Cepheid Xpert HIV-1 Qual
- Cepheid Xpert MRSA NxG
- Cepheid Xpert MRSA/SA Blood Culture
- Cepheid Xpert MRSA/SA SSTI
- Cepheid Xpert MTB/RIF
- Cepheid Xpert SA Nasal Complete
- Cepheid Xpert vanA/vanB
- Cepheid Xpert Xpress Flu/RSV
- Cepheid Xpert Xpress SARS-CoV-2
- Qiagen QIAamp Viral RNA Mini Kit (250)
- Thermo Fisher Scientific Applied Biosystems AmpFLSTR Identifiler Plus PCR Amplification Kit
- Thermo Fisher Scientific Applied Biosystems MagMAX-96 Viral RNA Isolation Kit
- Thermo Fisher Scientific Invitrogen ERCC RNA Spike-In Mix
- Thermo Fisher Scientific Invitrogen ProcartaPlex Human Immune Response Panel, 80plex
- Thermo Fisher Scientific Invitrogen SuperScript III One-Step RT-PCR System with Platinum Taq DNA Polymerase
- Invitrogen SuperScript IV One-Step RT-PCR System with Platinum Taq DNA Polymerase
- Thermo Fisher Scientific Invitrogen SYBR Safe DNA Gel Stain
- Abbott GRAFTMASTER RX Coronary Stent Graft System (SD 3.5)
- Qiagen QIAamp DNA Mini Kit (250)
-
Medical LAB
- Eppendorf Multipette M4 - Multi-Dispenser Pipette
- Microgen D-125 Cleaner wipes
- BioRad Gel Releasers
- BioRad Genesis Cell Isolation System
- BioRad Centrifuge L
- BioRad BR-2000 Vortexer
- BioRad ZE5 Cell Analyzer
- BioRad Model 16K Microcentrifuge
- Metrohm Combustion Tube (AJ)
- Metrohm PTFE capillary 0.97 mm i.d. / 5 m
- Metrohm Pharm-A-line pump tubing to the peristaltic pump module
- Metrohm Weighing boats
- Agilent Technologies Cary 60 UV-Vis Spectrophotometer
- Thermo Scientific EvolutionPro UV-Vis Spectrophotometers
- Thermo Scientific M104 Muffle Furnace, standard model, with temperature controller and upper limit cut-out
- Thermo Scientific Multiskan SkyHigh Microplate Spectrophotometer
- Thermo Scientific ThermoFlex Recirculating Chillers
- Eppendorf BioFlo 320
- Eppendorf DASbox Mini Bioreactor System
- Eppendorf BioBLU 0.3f Single-Use Bioreactor
- Metrohm Ag/AgCl reference electrode with KCl
- Metrohm 905 Titrando
- Metrohm Flow-through measuring vessel
- Metrohm Exchange Unit
- Metrohm Sample beakers (120 mL)
- Metrohm Separate polymer membrane electrode, Ca
- Eppendorf SmartBlock 1.5 mL
- BioRad GelDoc Go Gel Imaging System with Image Lab Touch Software
- Metrohm Needles for MME
- Eppendorf CryoCube F440n Series - ULT Freezer
- Metrohm OMNIS Titrator Salt
- Metrohm PTFE piston
- Metrohm Inlet valve PEEK
- Metrohm Absorber tube to the Absorber Module
- Thermo Scientific CryoExtra High-Efficiency Cryogenic Storage Systems
- Thermo Scientific ThermoFlex Recirculating Chillers (LN)
- Thermo Fisher Scientific Invitrogen E-Gel Power Snap Electrophoresis System
- Thermo Scientific picoSpin 80 Series II NMR Spectrometer
- Metrohm Ion-selective electrode, Pb
- Eppendorf BioFlo 720
- ESCO CelCulture Incubator 50 L
- Metrohm Ion-selective Electrode F
- Metrohm iEcotrode Plus
- ESCO Versati Tabletop Ventilated Centrifuge
- Eppendorf MiniSpin - Mini Centrifuge
- ESCO Cytoculture Cytotoxic Class II Biohazard Safety Cabinet
- BioRad Mini-PROTEAN Tetra Cell, Mini Trans-Blot Module, and PowerPac Basic Power Supply
- Eppendorf Centrifuge 5910 Ri - Refrigerated Centrifuge (Rotor S-4x Universal)
- Metrohm 904 Titrando
- ESCO CelMate Incubator with UV Lamp, 50 L
- Metrohm PTFE Capillary 0.5 mm i.d.
- Metrohm Buffer solutions pH 4, 7 and 9
- Metrohm Metrosep C 4 Guard/4.0
- Thermo Fisher Scientific Sorvall Legend Micro 21R Microcentrifuge
- ESCO OrbiCult Incubator Benchtop Shaker - Refrigerated (19 mm)
- Eppendorf Centrifuge 5425 R - Microcentrifuge (Rotor FA -24X2)
- Metrohm Eco Titrator
- Thermo Scientific Finnpipette 2
- ESCO Airstream NS G4 Class II Type A2 Biological Safety Cabinet
- Eppendorf Bioprocess Autosampler
- PerkinElmer LAMBDA 1050+ UV/Vis/NIR Spectrophotometer
- Metrohm PTFE Tubing M8
- Mettler Toledo SevenExcellence pH meter S400
- Eppendorf BioFlo 120
- Metrohm Unitrode with Pt1000
- Metrohm Solvotrode
- Eppendorf CryoCube F101h - Ultra-low freezer
- Metrohm Filtration membrane reg. cellulose
- Metrohm Strand 2 M
- Eppendorf ThermoMixer C
- Metrohm Septum
- ESCO Isotherm Refrigerated Incubator, 170 L (Stainless Steel Exterior Cabinet)
- ESCO Lexicon II Ultra-low Temperature Freezer, 363 L
- Metrohm Combined pH glass Electrode
- ESCO Lexicon II Ultra-low Temperature Freezer, 714 L
- ART Pipette tips
- mySPIN 12 Mini Centrifuge
- Finntip Accessories
- MicroAmp PCR WORK accessories
- Hard-Shell 96-Well PCR Plates
- Applied Biosystems MicroAmp Optical 96-Well Reaction Plate
- Airstream Class II Type A2 Biological Safety Cabinet
- Specimen Collection Containers (Sterile Non-Sterile)
- Pipette ESR Westergren Method
- CelCulture CO2 Incubator
- Pasteur Pipette (sterile - Non-Sterile )
- Urine Container
- Petri dish
- Finnpipette F2 Variable Volume Pipettes
- Lexicon II Ultra - Low Temperature Freezer
- Thermo Scientific Nunclon Sphera Microplates
- Microseal 'B' PCR Plate Sealing Film
- 0.2 ml Flat PCR Tube 8-Cap Strips
- 0.2 ml 8-Tube PCR Strips without Caps
- ESCO Airstream Class II Type A2 Biological Safety Cabinet
- Airstream Class III Type A2 Biological Safety Cabinet
- BioRad ChemiDoc MP Imaging System
- BioRad ChemiDoc Imaging System
- BioRad PowerPac Universal Power Supply
- BioRad Trans-Blot SD System and PowerPac HC Power Supply System
- BioRad Trans-Blot Turbo Transfer System
- Eppendorf Centrifuge 5418 R - Microcentrifuge (Rotor FA-45-18-11)
- Eppendorf Centrifuge 5420 - Microcentrifuge (Rotor FA-24x2)
- Eppendorf Centrifuge 5430 - High-Speed Centrifuge (Rotor FA-45-30-11)
- Eppendorf Centrifuge 5430 R - High-Speed Centrifuge (Rotor FA-45-30-11)
- Eppendorf Centrifuge 5702 - Low-Speed Centrifuge (Rotor A-4-38)
- Eppendorf Centrifuge 5702 R - Low-Speed Centrifuge (Rotor A-4-38)
- Eppendorf Centrifuge 5804 - Benchtop Centrifuge (Rotor A-4-44)
- Eppendorf Centrifuge 5804 - Benchtop Centrifuge (Rotor S-4-72)
- Eppendorf Centrifuge 5804 R - Table Centrifuge (Rotor S-4-72)
- Eppendorf Centrifuge 5810 - Benchtop Centrifuge (Rotor A-4-62)
- Eppendorf Centrifuge 5810 - Benchtop Centrifuge (Rotor A-4-81)
- Eppendorf Centrifuge 5810 - Benchtop Centrifuge (Rotor S-4-104)
- Eppendorf centrifuge machineCentrifuge 5810 R - Benchtop Centrifuge (Rotor A-4-62)
- Eppendorf Centrifuge 5810 R - Benchtop Centrifuge (Rotor A-4-81)
- Eppendorf Centrifuge 5810 R - Benchtop Centrifuge (Rotor S-4-104)
- Eppendorf MiniSpin plus - Mini Centrifuge
- Thermo Fisher Scientific Fresco 17 Microcentrifuge
- Thermo Fisher Scientific Fresco 21 Microcentrifuge
- Thermo Fisher Scientific mySPIN 12 Mini Centrifuge
- Thermo Fisher Scientific Pico 17 Microcentrifuge
- Thermo Fisher Scientific Pico 21 Microcentrifuge
- Thermo Fisher Scientific Sorvall Legend Micro 17 Microcentrifuge
- Thermo Fisher Scientific Sorvall Legend Micro 21 Microcentrifuge
- Thermo Fisher Scientific Sorvall Legend X1 Centrifuge
- BioRad Mini-PROTEAN Tetra Vertical Electrophoresis Cell
- BioRad PowerPac Basic Power Supply
- BioRad Gene Pulser Xcell Total System
- BioRad MicroPulser Electroporator
- Thermo Fisher Scientific Invitrogen Qubit 4 Fluorometer, with WiFi
- Eppendorf CryoCube F570 Series - ULT Freezer
- Eppendorf CryoCube F740 Series - ULT Freezer
- ESCO Lexicon II Ultra-low Temperature Freezer
- Eppendorf CellXpert C170i CO2 Incubators.
- Eppendorf mAb-Discovery Bundle: Cell Culture Screening Solution
- Eppendorf New Brunswick Innova 40R - Benchtop Orbital Shaker
- Eppendorf New Brunswick Innova 42R - Stackable Incubator Shaker
- Eppendorf New Brunswick S41i - CO2 Incubator Shaker
- ESCO CelCulture CO2 Incubator 170L
- Thermo Fisher Scientific Forma Direct Heat CO2 Incubator
- Thermo Fisher Scientific Forma Series II Water-Jacketed CO2 Incubator
- Thermo Fisher Scientific Forma Steri-Cycle CO2 Incubator
- Thermo Fisher Scientific Forma Steri-Cycle i160 Dual CO2 Incubator
- Thermo Fisher Scientific Heracell VIOS 160i CO2 Incubator
- Metrohm 846 Dosing Interface
- Metrohm 865 Dosimat Plus
- Metrohm 876 Dosimat Plus
- Metrohm 876 Manual Titrator Plus
- Metrohm Eco Dosimat
- Olympus CKX53 Cell Culture Microscope
- Olympus CX23 Biological Microscopes
- Olympus CX33 Biological Microscope
- Olympus CX43 Biological Microscope
- Olympus Magnus Ch20i Research Microscope
- Eppendorf Biospectrometer Basic.
- Thermo Fisher Scientific NanoDrop One Microvolume UV-Vis Spectrophotometer
- Thermo Fisher Scientific NanoDrop OneC Microvolume UV-Vis Spectrophotometer
- Eppendorf MixMate
- Eppendorf ThermoMixer F 1.5
- Metrohm 728 Magnetic Stirrer without stand
- Metrohm 803 TI Stand with stirrer and pump
- Metrohm 831 KF Coulometer
- Metrohm 851 Titrando
- Metrohm 852 Titrando
- Metrohm 860 KF Thermoprep
- Metrohm 870 KF Titrino plus complete
- Metrohm 874 Oven Sample Processor
- Metrohm 890 Titrando with Touch Control
- Metrohm 901 Titrando KF Titrations
- Metrohm 902 Titrando
- Metrohm 906 Titrando
- Metrohm 907 Titrando
- Metrohm 915 KF Ti-Touch
- Metrohm 916 Food Ti-Touch
- Metrohm 916 Oil Ti-Touch
- Metrohm 916 Salt Ti-Touch
- Metrohm 916 Ti-Touch
- Metrohm 917 Coulometer
- Metrohm Automated coulometric KF titrations
- Metrohm Automated volumetric KF titration including sample preparation
- Metrohm Automated volumetric KF titrations
- Metrohm Eco Coulometer
- Metrohm Eco KF Titrator
- Metrohm Eco Titrator Acid/Base
- Metrohm Eco Titrator Biogas
- Eco Titrator Oil
- Metrohm Eco Titrator Redox
- Metrohm Eco Titrator Salt
- Metrohm OMNIS Advanced Titrator
- Metrohm OMNIS Basic Titrator
- Metrohm OMNIS Dosing Module
- Metrohm OMNIS Professional Titrator
- Metrohm OMNIS Titration Module
- Metrohm OMNIS Titrator Food
- Metrohm OMNIS Titrator KF
- Metrohm OMNIS Titrator Oil
- Metrohm OMNIS Titrator without function license
- Metrohm Dosing Unit
- Metrohm Glass Cylinder Unit
- Eppendorf SmartBlock 2.0 mL
- Metrohm 2 mm platinum electrode tip for CVS
- Metrohm 3-way stopper with antidiffusion valve
- Metrohm 5-ring conductivity measuring cell c = 0.7 cm-1 with Pt1000 (fixed cable)
- Metrohm 50 m spacer for the Wall-Jet cell
- Metrohm 800 Dosino
- Metrohm 807 Dosing Unit ETFE 20 mL
- Metrohm 854 iConnect
- Metrohm 913 pH Meter
- Metrohm 940 Professional IC Vario TWO/SeS/PP
- Metrohm Adapter sleeve, MSM
- Metrohm Adsorber Complete
- Metrohm Adsorber tube for Dosing Unit
- Metrohm Adsorber Tube with Tubing Nipple
- Metrohm Adsorption cartridge
- Metrohm Ag Titrode
- Metrohm Ag working Electrode
- Metrohm Air tube for oil measurements
- Metrohm Al foil
- Metrohm Antidiffusion tip
- Metrohm Antidiffusion valve
- Metrohm Aquatrode Plus with Pt1000 (fixed cable)
- Metrohm Aspiration Filter
- Metrohm Aspiration Tubing
- Metrohm Au Working Electrode
- Metrohm Ball stopper / B-14/(15)
- Metrohm Biotrode
- Metrohm Bottle Attachment
- Metrohm Bottle cap, multi-use
- Metrohm Bottle PE
- Metrohm Buffer solution pH 4 (500 mL)
- Metrohm Buffer solution pH 7 (500 mL)
- Metrohm Buffer solution pH 9 (500 mL)
- Metrohm Cable Strand
- Metrohm Cable USB A mini-DIN 8-pin
- Metrohm Cannula for gas feeding
- Metrohm Clip for SGJ 14/15
- Metrohm CO2 adsorption cartridge CW
- Metrohm Combined Ag ring electrode
- Metrohm Combined Polymer Membrane Electrode Ca
- Metrohm Combined polymer membrane electrode, K
- Metrohm Combined polymer membrane electrode, NO3
- Metrohm Combined Pt ring electrode
- Metrohm Complete LL-Ag/AgCl(gel) reference electrode
- Metrohm Conductivity measuring cell c = 0.1 cm-1 with Pt1000 (fixed cable)
- Metrohm Conductivity measuring cell c = 1.6 cm-1 with Pt1000 (fixed cable)
- Metrohm Conductivity standard 100 S/cm, 5 x 30 mL
- Metrohm Consumable Kit OMNIS Gripper
- Metrohm Coupling 2 x UNF 10/32
- Metrohm Coupling M8 inner
- Metrohm Cylinder unit Eco
- Metrohm Cylinder unit OMNIS special, 10 mL
- Metrohm dEcotrode Plus
- Metrohm Dis-Cover lid for OMNIS 120 mL sample beaker, 16 pieces
- Metrohm Dosing tip / M8 thread
- Metrohm Double Pt ring electrode for volumetry
- Metrohm Double Pt-wire electrode for coulometry
- Metrohm Double Pt-wire electrode for volumetry
- Metrohm Drip Pan for 789/ 815/ 855
- Metrohm Driving axle for rotating disk electrode (RDE)
- Metrohm Driving axle for rotating disk electrode (RDE) with mercury contact
- Metrohm Driving Belt
- Metrohm Dust filter
- Metrohm Ecotrode Gel
- Metrohm Ecotrode Plus
- Metrohm Electrode cable for plug in head U/plug F
- Metrohm Electrode Cable plug-in Head G
- Metrohm Electrode Cable-F
- Metrohm Electrode Holder
- Metrohm Electrolyte 3 mol/L KCl (250 mL)
- Metrohm Electrolyte NH4NO3 1 mol/L (50 mL)
- Metrohm EtOH-Trode
- Metrohm Exchange Unit Base Plate
- Metrohm FEP Tubing M6
- Metrohm Flat stopcock
- Metrohm Flexible Drive Shaft for Stirrer
- Metrohm Foam Barrier
- Metrohm Four-way micro dosing tip
- Metrohm Generator Electrode
- Metrohm GL 18 screw cap with hole
- Metrohm Glass Cylinder
- Metrohm Heating tube stopper
- Metrohm Heating tubing
- Metrohm Holder for Absorber Tubing
- Metrohm iAg Titrode
- Metrohm IC Conductivity Detector
- Metrohmv Inlet valve (metal-free)
- Metrohm Inline filter 2 m
- Metrohm Ion-Selective Electrode Cl
- Metrohm Ion-selective Electrode Cu
- Metrohm Ion-Selective Electrode I
- Metrohm iPt Titrode
- Metrohm iSolvotrode
- Metrohm iUnitrode with Pt1000
- Metrohm KF Adsorber Tube
- Metrohm KF Adsorber Tube for Coulometer Cell
- Metrohm KF Inlet and Aspiration Tube
- Metrohm KF titration vessel / 80-250 mL / coulometric
- Metrohm KF titration vessel lid with 5 openings
- Metrohm KF titration vessel with 2 lateral openings / 80-250 mL / coulometric
- Metrohm Light protection for glass cylinder of 806 Exchange units
- Metrohm LL ISE Reference Electrode
- Metrohm Measuring vessel cover for stability measuring instruments
- Metrohm Mercury drop capillary (non-silanized) for MME
- Metrohm Mercury drop capillary (silanized) for MME
- Metrohm Metrosep A Supp 17
- Metrohm Metrosep A Supp 17 Guard/4.0
- Metrohm Metrosep A Supp 5
- Metrohm Metrosep A Supp 5 - 250
- Metrohm Metrosep A Supp 5 Guard/4.0
- Metrohm Metrosep C 4 - 250/4.0
- Metrohm Metrosep C 6
- Metrohm Metrosep C 6 Guard/4.0
- Metrohm Metrosep C Supp 1 - 250/4.0
- Metrohm Metrosep Organic Acids - 250/7.8
- Metrohm Metrosep Organic Acids Guard/4.6
- Metrohm Metrosep RP 2 Guard/3.5
- Metrohm Metrosep RP 3 Guard HC/4.0
- Metrohm Micro Electrode
- Metrohm Micro tip M6 thread
- Metrohm Mini USB Adapter Cable
- Metrohm Molecular Sieve
- Metrohm MSM Rotor A
- Metrohm Needle holder for MME pro
- Metrohm Needle holder with Luer lock
- Metrohm Needle with Luer Connector
- Metrohm NIRS XDS lab spare lamp
- Metrohm Numerical USB keypad
- Metrohm O-Ring
- Metrohm OMNIS electrode holder
- Metrohm Optrode
- Metrohm Outlet valve (metal-free)
- Metrohm PEEK Sample Loop
- Metrohm Outlet valve PEEK
- Metrohm Overflow protection for Ti Stand
- Metrohm PEEK capillary 0.5 mm i.d.
- Metrohm PharMed pump tubing with 3-stopper
- Metrohm pHit Kit
- Metrohm Pipetting tubing / 10 mL with holder for sample processors
- Metrohm Piston seal
- Metrohm Piston tool for Eco Dosimat/Eco Titrator
- Metrohm Polystyrene measuring vessel for stability measurements
- Metrohm PP Eluent Bottle
- Metrohm Pressure screw 5x
- Metrohm Protective cover for 900 Touch Control
- Metrohm Pt Titrode
- Metrohm PTFE Sleeve
- Metrohm PTFE Tubing
- Metrohm PTFE Tubing 2.5 m
- Metrohm Pump Tubing
- Metrohm Pump tubing LFL with 3 stoppers
- Metrohm PVC Tubing
- Metrohm Quartz Boat (AJ)
- Metrohm Robotic arm with transfer head for 786 Swing Head, right swinging
- Metrohm Sample beaker (250 mL)
- Metrohm Sample glass, 6 mL, 1000 pieces
- Metrohm Sample needle made of zirconium oxide with PEEK tip
- Metrohm Sample Rack
- Metrohm Sample vessel 11 mL
- Metrohm Septum Piercing Needle
- Metrohm Septum Stopper
- Metrohm Septum stopper for 6014050X0 titration vessel
- Metrohm Set of Seals
- Metrohm Set of seals for Karl Fischer titration cell
- Metrohm SGJ stopper SGJ 14
- Solitrode with Pt1000
- Metrohm Silicone seal
- Metrohm Silicone tubing 0.22 m
- Metrohm Sleeve with SGJ 14/12 mm
- Metrohm Solvotrode easyClean
- Metrohm Spare Filter for Inline Filter
- Metrohm Spare filter for RP 2 Guard/3.5, 10 pieces
- Metrohm Spray nozzle
- Metrohm Stand plate for 912/913/914
- Metrohm Stirring Bar
- Metrohm Stirring Propeller
- Metrohm Stopper
- Metrohm Stopper with perforation
- Metrohm Storage solution
- Metrohm Strand 1 M
- Metrohm Substrate made of quartz for liquid samples
- Metrohm Support rod / 300 mm
- Metrohm Syringe 5 mL
- Metrohm T connector CIC
- Metrohm TEABr 0.4 mol/L in ethylene glycol (250 mL)
- Metrohm Thread Adapter
- Metrohm Thread adapter M8 outer
- Metrohm Threaded Stopper M6
- Metrohm Threaded Stopper M8
- Metrohm Tiamo 2.5 Light CD: 1 license
- Metrohm Titration Head, 3 x SGJ 14
- Material Titration tip
- Metrohm Titration vessel
- Metrohm Titration vessel with thermostat jacket / 50-150 mL
- Metrohm Tube
- Metrohm Tubing cartridge
- Metrohm Vial PP 2.5 mL
- Metrohm Viscotrode
- Metrohm Zirconium Oxide Piston
- Metrohm Ag Titrode with Ag2S coating
- Metrohm Electrode Cable-H
- ESCO Airstream Class II, B2 Biological Safety Cabinet
- ESCO Airstream Class III Biological Safety Cabinet
- ESCO Airstream Gen 3 Horizontal Laminar Flow Cabinet
- ESCO Airstream Gen 3 Vertical Laminar Flow Cabinet
- ESCO Versati Micro Refrigerated Centrifuge
- ESCO Versati Micro Ventilated Centrifuge
- ESCO Versati Tabletop Refrigerated Centrifuge
- ESCO Lexicon II Ultra-low Temperature Freezer, 480 L
- ESCO Lexicon II Ultra-low Temperature Freezer, 597 L
- ESCO Ascent Max Ductless Fume Hood - Standard
- ESCO Frontier Duo Fume Hood, 1.2 m
- ESCO CelCulture CO2 Incubator with High-Heat Sterilization, 170 L
- ESCO CelCulture CO2 Incubator with UV Lamp, 170 L
- ESCO CelMate Incubator, 170L
- ESCO Isotherm Refrigerated Incubator, 170 L
- ESCO Isotherm Refrigerated Incubator, 240 L
- ESCO Lexicon II Ultra-low Temperature Freezer, 363 L (5 inner door)
- ESCO Isotherm Forced Convection Lab Oven, 110 L
- ESCO OrbiCult Incubator Benchtop Shaker, Non-Refrigerated (19 mm)
- Centrifuge 5804 R - Benchtop Centrifuge (Rotor A-4-44)
- Thermo Fisher Scientific Tencell Cuvettes
- Thermo Fisher Scientific Nicolet iS20 FTIR Spectrometer
-
Medical Surgicals
- Medtronic NC Sprinter RX Noncompliant Balloon Catheter (BD 3.50)
- Medtronic NC Sprinter RX Noncompliant Balloon Catheter (BD 4.00)
- Abbott PACEL Bipolar Pacing Catheter, Ventricular Pacing with Right Heart Curve
- Medtronic Everest30 Survival Kit
- Medtronic Euphora Semicompliant Balloon Dilatation Catheter (BD 3.25)
- Medtronic NC Sprinter RX Noncompliant Balloon Catheter (BD 3.00)
- Abbott NC Trek Rx Coronary Dilation Catheter (BD 3.00)
- Abbott NC Trek Rx Coronary Dilation Catheter (BD 2.00)
- ASAHI Tornus Coronary Microcatheters
- Abbott PACEL BIPOLAR PACING CATHETERS VENTRICULAR PACING WITH RIGHT HEART CURVE Torque Control
- ASAHI Corsair Pro
- Medtronic-Covidien EEA Hemorrhoid and Prolapse Stapler Set with DST Series Technology
- Medtronic Euphora Semicompliant Balloon Dilatation Catheter (BD 2.25)
- Medtronic Everest30 Inflation Device and 3-way stopcock
- Terumo RUNTHROUGH NS HYPERCOAT Coronary Guidewire
- Medtronic Resolute Onyx DES Drug-eluting Stent (SD 3.50)
- Abbott PACEL BIPOLAR PACING CATHETERS VENTRICULAR PACING 60 DEGREE CURVE Torque Control
- Abbott NC Trek Rx Coronary Dilation Catheter (BD 2.75)
- Medtronic NC Euphora Noncompliant Balloon Dilatation Catheter
- Abbott Viatrac 14 Plus Peripheral Dilatation Catheter
- Abbott TREK Coronary Dilatation Catheter (BD 2.50)
- Medtronic Sprinter Legend 1.25 SuperCrosser Semicompliant Balloon Dilatation Catheter
- Abbott MINI TREK Coronary Dilatation Catheter (BD 2.00)
- Medtronic Euphora Semicompliant Balloon Dilatation Catheter (BD 2.50)
- Abbott PACEL Bipolar Pacing Catheter, Right Heart Curve
- Medtronic Euphora Semicompliant Balloon Dilatation Catheter (BD 2.75)
- Abbott NC Trek Rx Coronary Dilation Catheter (BD 4.00)
- Abbott NC Trek Rx Coronary Dilation Catheter (BD 4.50)
- ASAHI Caravel
- Abbott PACEL Bipolar Pacing Catheter, Ventricular Pacing 60 Degree Curve
- Medtronic-Covidien Parietene DS Composite Mesh, 15 cm round
- ASAHI SION blue
- Medtronic Export AP Aspiration Catheters
- ASAHI Gaia Next 1
- Abbott MINI TREK Coronary Dilatation Catheter (BD 1.20)
- Abbott MINI TREK II OTW Coronary Dilatation Catheter (BD 1.50)
- Abbott NC Trek Rx Coronary Dilation Catheter (BD 3.25)
- Abbott NC Trek Rx Coronary Dilation Catheter (BD 2.25)
- Abbott NC Trek Rx Coronary Dilation Catheter (BD 3.50)
- Abbott Herculink Elite Renal Stent System (SD 4.5)
- Abbott Herculink Elite Renal Stent System (SD 6.0)
- Medtronic - Covidien LigaSure Maryland Jaw Thoracic Sealer/ Divider (30 cm)
- Medtronic-Covidien Appose ULC skin stapler
- Medtronic-Covidien EEA Circular Stapler with DST Series Technology (LS 25mm)
- Medtronic-Covidien EEA Circular Stapler with DST Series Technology (LS 28mm)
- EEA Circular Stapler with DST Series Technology (LS 31mm)
- Medtronic-Covidien Endo GIA 45 mm Articulating Extra Thick Reload
- Medtronic-Covidien Endo GIA 60 mm Articulating Extra Thick Reload
- Medtronic-Covidien Endo GIA Reloads with Tri-Staple Technology
- Medtronic-Covidien GIA Stapler with DST Series Technology
- Medtronic-Covidien GIA Stapler with Tri-Staple Technology, 60 mm Medium/Thick Cartridge
- Medtronic-Covidien LigaSure Maryland Jaw Open and Laparoscopic Sealer/Divider with Nano-coating
- Medtronic-Covidien Premium single-use skin staple remover
- Medtronic - Covidien AbsorbaTack Fixation Device
- Medtronic - Covidien ProTack 5 mm Instrument
- Medtronic-Covidien Parietene DS Composite Mesh, 25 x 20 cm
- Medtronic-Covidien Parietene DS Composite Mesh, 30 x 20 cm
- Medtronic-Covidien Parietex Optimized Composite (PCOx) Mesh
- ASAHI FUBUKI 043 Intermediate Catheter
- ASAHI FUBUKI 6F
- ASAHI FUBUKI 7F
- ASAHI FUBUKI 8F
- ASAHI FUBUKI Long Sheath, 6Fr
- ASAHI FUBUKI Long Sheath, 7Fr
- ASAHI CHIKAI 10
- ASAHI CHIKAI black 14 soft tip
- ASAHI FUBUKI XF 6Fr Long Sheath
- Terumo Accuforce PTCA Dilatation Catheter (BD 2.25)
- Terumo Accuforce PTCA Dilatation Catheter (BD 2.50)
- Terumo Accuforce PTCA Dilatation Catheter (BD 2.75)
- Terumo Accuforce PTCA Dilatation Catheter (BD 3.00)
- Terumo Accuforce PTCA Dilatation Catheter (BD 3.25)
- Terumo Accuforce PTCA Dilatation Catheter (BD 3.50)
- Terumo Accuforce PTCA Dilatation Catheter (BD 3.75)
- Terumo Accuforce PTCA Dilatation Catheter (BD 4.00)
- Terumo Accuforce PTCA Dilatation Catheter (BD 4.50)
- Terumo Angio-Seal VIP Vascular Closure Device
- Terumo Eliminate Aspiration Catheter
- Terumo Finecross MG Coronary Micro-Guide Cathether
- Terumo GLIDECATH Hydrophilic Coated Catheter 5Fr
- Terumo Progreat Micro Catheter System
- Terumo Ryujin Plus PTCA Dilatation Catheter (BD 2.50)
- Terumo Radifocus Optitorque Angiographic Catheter
- Terumo Radifocus Optitorque Angiographic catheter (CR- 4.00)
- Terumo Runthrough NS Intermediate
- Terumo Ryujin Plus OTW PTCA Dilatation Catheter (BL 15)
- Terumo Ryujin Plus OTW PTCA Dilatation Catheter (BL 20)
- Terumo Ryujin Plus PTCA Dilatation Catheter (BD 2.25)
- Terumo Ryujin Plus PTCA Dilatation Catheter (BD 2.75)
- Terumo Ryujin Plus PTCA Dilatation Catheter (BD 3.00)
- Terumo Tazuna PTCA Dilatation Catheter (RX) (BD 2.00)
- Terumo Tazuna PTCA Dilatation Catheter (RX) (BD 2.25)
- Terumo Tazuna PTCA Dilatation Catheter (RX) (BD 2.50)
- Abbott Emboshield NAV6 Embolic Protection System
- Abbott HI-TORQUE ALL STAR Guide Wires
- Abbott HI-TORQUE BALANCE HEAVYWEIGHT Guide Wire
- Abbott HI-TORQUE BALANCE MIDDLEWEIGHT UNIVERSAL II Guide Wire
- Abbott HI-TORQUE BALANCE MIDDLEWEIGHT UNIVERSAL Guide Wire
- Abbott HI-TORQUE BALANCE MIDDLEWEIGHT Guide Wire
- AbbottHI-TORQUE BALANCE MIDDLEWEIGHT Guide Wire (HC)
- Abbott Hi-Torque Command 18 LT Guide Wires
- Abbott Hi-Torque Command 18 ST Guide Wires
- Abbott Hi-Torque Command ES Guide Wires
- Abbott HI-TORQUE CROSS-IT 100XT Guidewire (190 cm)
- Abbott HI-TORQUE CROSS-IT 100XT Guidewire (300 cm)
- Abbott HI-TORQUE CROSS-IT 200XT Guide Wires
- HI-TORQUE CROSS-IT 300XT Guide Wires
- Abbott HI-TORQUE FLOPPY II Guide Wire (190 cm)
- Abbott HI-TORQUE FLOPPY II Guide Wire (300 cm)
- Abbott HI-TORQUE IRONMAN Guide Wires (190 cm)
- Abbott HI-TORQUE IRONMAN Guide Wires (300 cm)
- Abbott HI-TORQUE PILOT 150 Guide Wires (190 cm)
- Abbott HI-TORQUE PILOT 200 Guide Wires
- HI-TORQUE PILOT 50 Guide Wires
- Abbott HI-TORQUE POWERTURN FLEX Guide Wires (300 cm)
- Abbott HI-TORQUE POWERTURN Guide Wires (190 cm)
- Abbott HI-TORQUE VERSATURN Guide Wire
- Abbott HI-TORQUE WHISPER ES Guide Wires
- Abbott HI-TORQUE WHISPER MS Guide Wires
- Abbott TigerWire Steerable Guidewire
- ASAHI CONFIANZA PRO 8-20
- ASAHI Conquest Pro
- ASAHI Conquest Pro 12
- ASAHI EXTENSION 165
- ASAHI FIELDER
- ASAHI FIELDER FC Guidewire
- ASAHI FIELDER XT
- ASAHI FIELDER XT-A
- ASAHI FIELDER XT-R
- ASAHI Gaia Series
- ASAHI GRAND SLAM
- ASAHI Miracle 3/ MIRACLEbros 3
- ASAHI MIRACLEBROS 12
- ASAHI MIRACLEBROS 6
- ASAHI RG3
- ASAHI Rinato/PROWATER
- ASAHI SION blue ES
- ASAHI SION black, 190 cm
- ASAHI SION PTCA GW 190 cm, Straight
- ASAHI SUOH 03
- ASAHI ULTIMATEbros 3
- Asahi Astato XS 40 0.014 inch
- Medtronic Cougar LS Guidewire
- Terumo Radifocus Guide Wire M Standard Type
- Terumo Radifocus Guide Wire M Stiff Type
- Terumo Runthrough NS Extra Floppy
- Terumo Runthrough NS Floppy
- Terumo Runthrough NS Hypercoat
- Terumo Radifocus Introducer II Standard Kit A
- Terumo Radifocus Introducer II Standard Kit B
- Abbott Absolute Pro Peripheral Self-Expanding Stent System
- Abbott GRAFTMASTER RX Coronary Stent Graft System (SD 2.8)
- Abbott GRAFTMASTER RX Coronary Stent Graft System (SD 4.0)
- Abbott GRAFTMASTER RX Coronary Stent Graft System (SD 4.5)
- GRAFTMASTER RX Coronary Stent Graft System (SD 4.8)
- Abbott RX Acculink Carotid Stent System
- Abbott Supera Peripheral Stent
- Perclose ProGlide Suture-Mediated Closure (SMC) System
- Abbott Perclose ProStyle Suture-Mediated Closure And Repair System
- Medtronic - Covidien Polysorb Braided Absorbable Sutures
- Medtronic-Covidien Shiley Flexible Tracheostomy Tube With TaperGuard Cuff, Disposable Inner Cannula
- Abbott HI-TORQUE BALANCE MIDDLEWEIGHT Guide Wire (300cm)
-
Life Sciences
- Agilent Technologies 7850 ICP-MS Instrument
- PerkinElmer QSight 210 LC/MS/MS System
- Waters Corp GlycoWorks RapiFluor-MS N-Glycan Script Starter Kit-Automation
- Waters Corp 24-well Collection Plate, 10 mL Square well, 1/pk
- Mettler Toledo Melting Point System MP80
- Analytikjena Multi X 2500 AOX/TOX, EOX, POX analyzer
- Mettler Toledo TOC Sensor 6000i
- Thermo Scientific Element Series HR-ICP-MS
- Thermo Scientific iCAP RQplus ICP-MS
- Waters Corp Sep-Pak 500 mg Alumina N 3 cc Vac Cartridge and Sorbent per Cartridge
- Waters Corp wwPTFE Acrodisc Mini Syringe Filter, 0.45 um 13 mm 100/Pk
- Metrohm i-Raman EX Portable Raman Spectrometer
- Thermo Scientific 5014i Beta Continuous Ambient Particulate Monitor
- Thermo Scientific RadEye GN+ Gamma Neutron Pager
- Mettler Toledo Technical buffer pH 10.00 250mL Bottle
- Shimadzu EPMA - 1720 Electron Probe Micro Analyzer
- Thermo Scientific PDM3700 Personal Dust Monitor
- PerkinElmer Spectrum Two FT-IR LiTaO3 Detector Spectrometer
- Bruker Invenio Confocheck Protein Analysis FTIR
- PerkinElmer PinAAcle 500 Flame Atomic Absorption Spectrometer
- Mettler Toledo Halogen Moisture Analyzer HS153
- Bruker Bravo Handheld Raman Spectrometer
- PerkinElmer Avio 550 Max ICP-OES Oils Configuration
- PerkinElmer Avio 550 Max ICP-OES Scott/Cross-Flow Configuration
- PerkinElmer Spectrum Two Nutraceutical & TCM System
- PerkinElmer Spectrum Two Pharmaceutical System
- Mettler Toledo Technical buffer pH 4.01 30x20mL Sachets
- PerkinElmer Spotlight 200i FT-IR Microscopy System with Spectrum Two
- PerkinElmer Spotlight 400 FT-IR Imaging System
- Shimadzu EPMA - 8050G Electron Probe Micro Analyzer
- Mettler Toledo C31 StandardLine Checkweigher
- Waters Corp Protein-Pak Bulk Epoxy Activated Affinity Material, 25 g
- Thermo Scientific Model 49i Ozone Analyzer
-
Medical IVD
- Contact Us
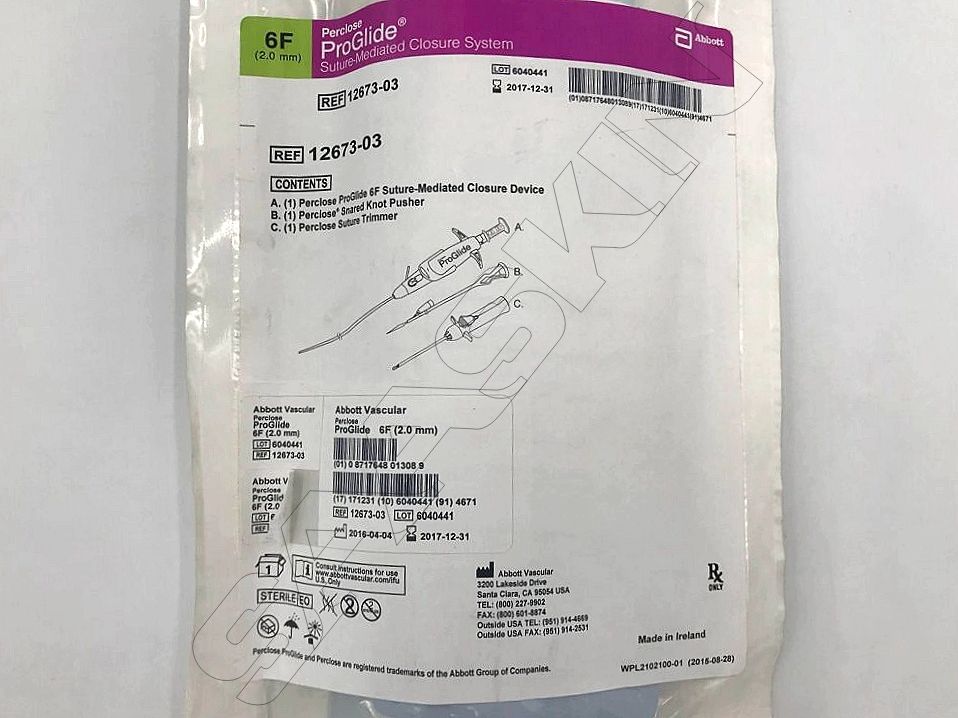
- Home
- Products
- Medical Surgicals
- Perclose ProGlide Suture-Mediated Closure (SMC) System
Perclose ProGlide Suture-Mediated Closure (SMC) System
Product Details:
- Shelf Life 3 years
- Accuracy Consistent suture placement and vessel closure
- Instruments Type Vascular Closure Device
- Features Single-handed deployment, Minimally invasive, Ergonomic design
- Storage Instructions Store in a dry, room temperature environment
- Measurement Range 6F to 8F femoral vein/artery access sites
- Function Achieves percutaneous suture closure of vessel access sites
- Click to View more
-
Share Your Product:
X
Perclose ProGlide Suture-Mediated Closure (SMC) System Price And Quantity
Perclose ProGlide Suture-Mediated Closure (SMC) System Product Specifications
- Medical Grade Polymer and Stainless Steel
- 3 years
- Consistent suture placement and vessel closure
- 30 cm (L)
- Yes
- None (Manual Operation)
- Vascular Closure Device
- Silent / Negligible
- Vascular Access Site Closure
- Single-handed deployment, Minimally invasive, Ergonomic design
- Approx. 55 grams
- No
- White/Blue
- Suture-Mediated Vascular Closure
- 6F to 8F femoral vein/artery access sites
- Achieves percutaneous suture closure of vessel access sites
- Manual
- Store in a dry, room temperature environment
- Yes
- Hospital / Clinical
- New
- Suture-Mediated Closure System
Product Description
| Brand | Abbott |
| Model | ProGlide |
| Pack Size | 10 nos |
| CAT No | 12673-03 |
Perclose ProGlide SMC System delivers a secure, non-masking percutaneous suture to the access site that promotes primary healing1 and has no re-access restrictions. This system has the broadest arterial and venous indication*; it can be utilized for 5-21F2 (Max. 26F OD3) arterial sheaths and 5-24F2 (Max. 29F OD3) venous sheaths.
Advanced Suture-Mediated Closure Technology
The Perclose ProGlide System employs innovative suture-mediated technology for the effective closure of vascular access sites. Designed for use with 6F to 8F sheaths, it delivers reproducible results, minimizing tissue trauma and expediting patient recovery. This device is ideal for hospitals and clinical settings prioritizing precision and patient safety.
Simple, Manual Operation with Ergonomic Benefits
Engineered for manual use, the Perclose ProGlide System is completely portable, requires no external power, and maintains a quiet working environment. Its ergonomic structure facilitates single-handed deployment, improving workflow for trained healthcare professionals while ensuring consistent and secure vessel closure.
Safe, Sterile, and Ready for Use
Each Perclose ProGlide device is individually wrapped in sterile, peel-open trays, ensuring readiness for immediate clinical application. It is latex-free to reduce allergic risks, and training is highly recommended for optimal and safe usage. After deployment, the device is disposed of in biohazardous waste following hospital protocols.
FAQ's of Perclose ProGlide Suture-Mediated Closure (SMC) System:
Q: How is the Perclose ProGlide SMC System deployed for vascular access site closure?
A: The device is inserted following vascular access, and a suture is deployed via a manual, single-handed operation. The polypropylene suture is then cut to achieve mechanical closure of the vessel entry site.Q: What are the compatibility requirements for the Perclose ProGlide System?
A: It is compatible with 6 French to 8 French introducer sheaths and intended for adult patients requiring femoral vein or artery closure.Q: When should clinicians dispose of the Perclose ProGlide device?
A: After single use, the Perclose ProGlide SMC System should be discarded as biohazardous waste according to institutional disposal protocols.Q: Where should the Perclose ProGlide SMC System be stored prior to use?
A: Store the device in a dry, room-temperature environment to maintain sterility and maximize shelf life, which extends up to 3 years if properly stored.Q: What training is needed to operate the Perclose ProGlide SMC System?
A: Device-specific training for operators is strongly recommended to ensure proper technique, safety, and effective closure outcomes.Q: What benefits does the Perclose ProGlide SMC System offer over traditional closure methods?
A: It provides minimally invasive, fast, and reliable closure with single-handed deployment, reducing procedure time and promoting patient comfort during recovery.Q: How does the Perclose ProGlide achieve accuracy in suture placement?
A: The device is engineered for consistent and reliable suture placement, leveraging its ergonomic design and specialized mechanism to deliver precise vessel closure every time.Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
Name
Comapny Name
Phone Number
Email Id
City / State
Confirm Your Requirement
Verification Code
Did not receive yet?
Resend OTP

Youre Done!
We have received your requirements and will reply shortly with the best price.
Products You May Like
Other Products in 'Medical Surgicals' category
×
Add a mobile number to receive call from "Saaskin Corporation Private Limited."
×
Enter OTP
×
Share additional details for a quick response
×
Thank You!
Thank You for your valuable time. We have received your details and will get back to you shortly.
×
Get a price quote for Perclose ProGlide Suture-Mediated Closure (SMC) System
×
- Saaskin Corporation Private Limited.
GST : 33AAZCS6003M1ZU
- 275/184, 1st Floor, Office No: 2, Golden Enclave, Periyar E V R Salai, P.H. Road, Kilpauk,Chennai - 600010, Tamil Nadu, India
- Mobile : 0091 9840819191 / 9940116677
- Phone:07971189171
- Mr Rajesh H (Business Operations Manager)
- Mobile:07971189171
|
-
 Accepts only Export inquiries
Accepts only Export inquiries
 info@saaskin.com
info@saaskin.com


 |
SAASKIN CORPORATION PRIVATE LIMITED
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |
✕
Contact Us
Name
Comapny Name
Phone Number
Email Id
City / State

OTP Verification
Verification Code
Did not receive yet?
Resend OTP

Thank you!
We have received your requirements
For an immediate response, please call this
number 07971189171
✕
Tell us about your requirement

Price:
Quantity
Select Unit
Additional detail
Mobile number
Email
Name
Comapny Name
Phone Number
Email Id
City / State
Confirm Your Requirement
Verification Code
Did not receive yet?
Resend OTP

Youre Done!
We have received your requirements and will reply shortly with the best price.
Products You May Like









