Terumo Accuforce PTCA Dilatation Catheter (BD 2.25)
Product Details:
- Instruments Type Interventional Cardiology Device
- Measurement Range Balloon Diameter: 2.25 mm
- Accuracy High positional accuracy of balloon
- Usage Type Single-use
- Storage Instructions Store in a cool, dry place away from direct sunlight
- Features Flexible tip design; Radiopaque marker bands; Smooth balloon transition
- Shelf Life Up to 3 years (unopened)
- Click to View more
X
Terumo Accuforce PTCA Dilatation Catheter (BD 2.25) Price And Quantity
Terumo Accuforce PTCA Dilatation Catheter (BD 2.25) Product Specifications
- Yes
- Lightweight (Exact value not specified)
- Clear/Blue Coding
- Balloon Diameter: 2.25 mm
- No
- Yes
- Interventional Cardiology Device
- Semi-Compliant Balloon Technology
- Flexible tip design; Radiopaque marker bands; Smooth balloon transition
- Manual
- Polyamide / Pebax / Stainless Steel
- Coronary Angioplasty Procedure
- Catheter Length: 142 cm (usable length)
- Silent
- Single-use
- Balloon dilation of stenotic coronary arteries
- Store in a cool, dry place away from direct sunlight
- PTCA Dilatation Catheter
- Up to 3 years (unopened)
- High positional accuracy of balloon
- New
Product Description
| Brand | Terumo |
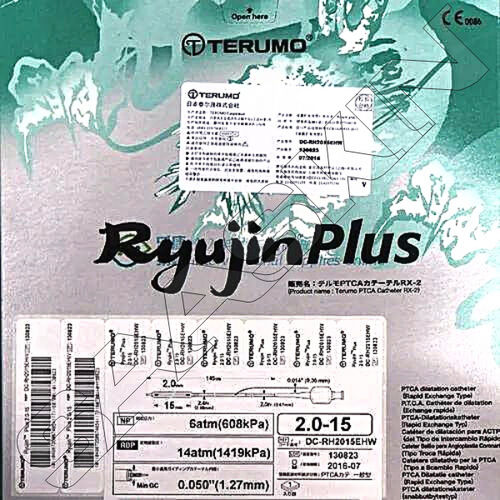
| Ballon Diameter (mm) | 2.25 |
| Ballon Length (mm) | 6, 8, 12 & 15 |
| CAT No. | DC-RM2206HSW, DC-RM2208HSW, DC-RM2212HHW & DC-RM2215HHW |
| Catheter Length (cm) | 110 |
The great balance between high pressure and high resistance, accurate dilatation and advanced deliverability. Accuforce is the latest generation of non-compliant PTCA balloons.
Advanced Semi-Compliant Balloon Technology
The Accuforce BD 2.25 features semi-compliant polyamide balloon technology, delivering controlled inflation and effective plaque dilation with minimal vessel trauma. Its soft tapered tip and smooth balloon transition enhance safety, while radiopaque marker bands allow for precise deployment under fluoroscopy.
Reliable Sterility and Packaging
Each Terumo Accuforce PTCA Dilatation Catheter is EO-sterilized and individually packed in a sterile, clear/blue-coded container. This ensures the highest level of safety for patients, with all devices supplied in new condition and ready for immediate use in interventional cardiology settings.
Designed for Seamless Clinical Workflow
Engineered for compatibility with standard PTCA guide catheters and 0.014-inch guidewires, the catheter's lightweight design ensures easy handling. The 142 cm usable length provides ample reach for coronary procedures, with a silent operation and high positional accuracy supporting real-time clinical performance.
FAQ's of Terumo Accuforce PTCA Dilatation Catheter (BD 2.25):
Q: How does the Terumo Accuforce PTCA Dilatation Catheter ensure accurate placement during coronary angioplasty?
A: The catheter features two radiopaque marker bands and a flexible, soft tapered tip, allowing clinicians to visualize and position the balloon precisely at the target site under fluoroscopy. This design promotes controlled and accurate balloon deployment.Q: What is the process for using the Accuforce BD 2.25 catheter in a coronary angioplasty procedure?
A: The catheter is introduced over a compatible 0.014-inch guidewire through a standard PTCA guide catheter. After reaching the target vessel, the balloon is inflated gently to the desired pressure (nominal: 8 atm, maximum: 14 atm) to dilate stenotic sections, followed by deflation and removal.Q: When should the Accuforce BD 2.25 catheter be used, and is it reusable?
A: The Accuforce BD 2.25 is intended for use during coronary angioplasty procedures to dilate narrowed coronary arteries. It is single-use only and should not be reused; each catheter is sterile and individually packed for one-time patient application.Q: Where can medical professionals source the Terumo Accuforce PTCA Dilatation Catheter in India?
A: The catheter is available through authorized dealers, distributors, exporters, manufacturers, suppliers, traders, and wholesalers across India, ensuring wide accessibility for healthcare facilities.Q: What are the primary benefits of choosing the Accuforce BD 2.25 for coronary interventions?
A: Key benefits include its semi-compliant balloon for controlled dilation, high positional accuracy, flexible design for smooth vessel navigation, and reliable sterile single-use packaging. These features minimize trauma and enhance procedural efficacy.Q: How should the Accuforce BD 2.25 catheter be stored to maintain sterility and shelf life?
A: Store the catheter in a cool, dry place away from direct sunlight. When unopened and correctly stored, it maintains sterility and usability for up to three years from the date of manufacture.Q: What safety features are integrated into the design of the Accuforce BD 2.25?
A: The catheter includes a soft tapered tip to reduce vessel trauma, semi-compliant balloon material for effective dilation, and radiopaque markers for visibility. It's sterilized via ethylene oxide and presented in tamper-proof, sterile packaging to protect patient safety.Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
Other Products in 'Medical Surgicals' category
 |
SAASKIN CORPORATION PRIVATE LIMITED
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |