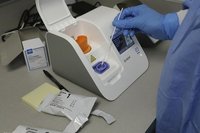
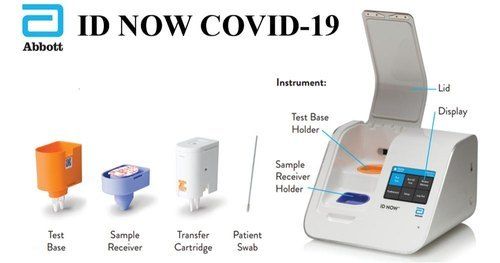
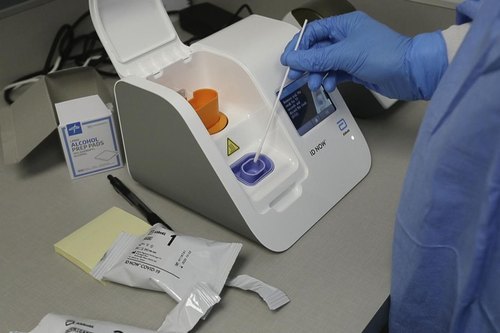
Abbott ID NOW Instrument
Product Details:
- Shelf Life N/A (Instrument Only)
- Features Rapid Results, Simple Workflow, Compact Design, User-Friendly Interface, Touchscreen Operation
- Function Qualitative Detection of Nucleic Acids
- Measurement Range Assay Dependent
- Accuracy >=95% (Assay Dependent)
- Storage Instructions Store in a clean, dry environment at room temperature
- Instruments Type Point-of-Care Diagnostic Device
- Click to View more
X
Abbott ID NOW Instrument Product Specifications
- Approx. 60W
- Molecular Diagnostic Instrument
- Rapid Molecular Testing (e.g., COVID-19, Influenza A/B)
- Isothermal Nucleic Acid Amplification
- Touchscreen LCD
- 19.1 x 21.6 x 15.2 cm
- AC Adapter
- No
- Clinical Laboratory, Point-of-Care
- White and Blue
- Yes
- 50/60 Hz
- Yes
- 100-240V AC, 50/60Hz
- Point-of-Care Diagnostic Device
- ~3.3 kg
- Qualitative Detection of Nucleic Acids
- Store in a clean, dry environment at room temperature
- Rapid Results, Simple Workflow, Compact Design, User-Friendly Interface, Touchscreen Operation
- Automatic
- Plastic, Metal Components
- Low
- New
- N/A (Instrument Only)
- >=95% (Assay Dependent)
- Assay Dependent
- Nasopharyngeal Swab, Throat Swab, Anterior Nares Swab
- Internal Memory Up to 1,000 Test Results
- Minimal, easy-to-clean design
- Capacitive Touch
- 5-13 minutes depending on assay
- 15-30°C
- USB, Ethernet
- 1 Test per Run
Abbott ID NOW Instrument Trade Information
- Chennai
- 2 Days
- carton
- Western Europe, Australia, North America, South America, Eastern Europe, Middle East, Africa, Central America, Asia
- All India
- FDA
Product Description
The ID NOW platform combines the benefits of speed and accuracy for the fastest molecular results in the market.
The ID NOW COVID-19 test returns positive results in 13 minutes or less to enable immediate clinical decisions during the first patient visit.
The easy to use ID NOW platform is designed for near-patient, point-of-care use in a variety of healthcare settings.
Product details
|
Direct Sample Type |
Throat Swab/Nasal Swab |
|
Communication Interface |
USB,Bluetooth |
|
Brand |
Abbott |
|
Model Name/Number |
ID Now |
|
Sample Capacity/Format |
Cartridge |
|
Volume Thermal Block Sample |
0.5 mL |
|
Scan time |
13 minutes |
|
Usage |
Molecular COVID-19 Test |
|
Storage Temperature |
Room Temperature |
Rapid and Reliable Molecular Testing
With the Abbott ID NOW Instrument, healthcare professionals can access fast and dependable molecular diagnostic results within minutes, supporting timely clinical decisions. Its cutting-edge isothermal amplification technology ensures high accuracy, while single-test throughput enables precise control and traceability for each patient sample. Built for use in both clinical laboratories and point-of-care settings, this device enhances testing capacity without compromising result quality.
Effortless Workflow and Operation
The ID NOW Instrument boasts a simple, streamlined workflow thanks to its intuitive capacitive touchscreen interface. Its compact, lightweight design saves valuable space while prioritizing user comfort. Minimal maintenance is required, allowing staff to focus on patient care. Data is stored securely within the device, making record management easy and efficient.
Flexible Sample Types and Connectivity
Compatible with nasopharyngeal, throat, and anterior nares swabs, the instrument supports diverse sampling needs. It facilitates seamless data integration with its USB and Ethernet ports, making it ideal for networked clinical environments. The white and blue device fits neatly on workbenches, operating reliably in a 15-30C environment, and its robust build ensures consistent, long-term performance.
FAQ's of Abbott ID NOW Instrument:
Q: How does the Abbott ID NOW Instrument perform rapid molecular tests?
A: The device utilizes isothermal nucleic acid amplification technology to detect pathogens in clinical samples. By maintaining a constant temperature, it rapidly amplifies nucleic acids, delivering qualitative results for infections such as COVID-19 or Influenza A/B in just 5-13 minutes.Q: What types of samples can be tested with the ID NOW Instrument?
A: This instrument accepts nasopharyngeal swabs, throat swabs, and anterior nares swabs, offering flexibility for different patient needs and sample collection techniques in clinical or point-of-care settings.Q: When are results available after running a test?
A: Depending on the assay performed, results are provided within 5 to 13 minutes, allowing healthcare workers to make timely, informed decisions about patient care.Q: Where can the Abbott ID NOW Instrument be used?
A: Thanks to its compact, lightweight, and portable design, the instrument is suitable for use in clinical laboratories, hospitals, clinics, and point-of-care locations throughout India. Its automatic operation and minimal noise also make it adaptable to various clinical environments.Q: What maintenance does the ID NOW Instrument require?
A: The device is engineered for minimal maintenance, featuring an easy-to-clean exterior and robust internal design, ensuring consistent and reliable operation with little downtime.Q: What are the benefits of using the Abbott ID NOW Instrument in healthcare settings?
A: Benefits include rapid and accurate results, user-friendly touchscreen interface, simple workflow, secure data storage, broad sample compatibility, low noise level, and automatic operation-all contributing to improved workflow efficiency and better patient care outcomes.Q: How is data from the tests managed and stored?
A: The ID NOW Instrument internally stores up to 1,000 test results, which can be easily accessed via its touchscreen interface. Data can be exported through USB or networked using Ethernet connectivity for secure integration into laboratory information systems.Tell us about your requirement

Price:
Quantity
Select Unit
- 50
- 100
- 200
- 250
- 500
- 1000+
Additional detail
Mobile number
Email
Other Products in 'Medical IVD' category
 |
SAASKIN CORPORATION PRIVATE LIMITED
All Rights Reserved.(Terms of Use) Developed and Managed by Infocom Network Private Limited. |